Chapter 20 the Representative Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Black Phosphorus As a New Lubricant
Friction 6(1): 116–142 (2018) ISSN 2223-7690 https://doi.org/10.1007/s40544-018-0204-z CN 10-1237/TH RESEARCH ARTICLE Black phosphorus as a new lubricant Wei WANG, Guoxin XIE*, Jianbin LUO* State Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China Received: 20 June 2017 / Revised: 18 October 2017 / Accepted: 07 December 2017 © The author(s) 2018. This article is published with open access at Springerlink.com Abstract: In recent years, a new 2D-layered material—black phosphorus (BP)—has been a rising star after the era of graphene owing to its high charge carrier mobility, tunable direct bandgap and unique in-plane anisotropic structure. With the development of the synthesis and modification methods of BP, its extensive applications, e.g., transistors, batteries and optoelectronics have emerged. In order to explore its full potential, research into the tribological properties of BP 2D-layered materials such as lubrication additives and fillers in self-lubricating composite materials would be not only of high scientific value but also of practical significance. In this work, recent advances on the friction and lubrication properties of BP nanosheets made by our group, including the micro-friction properties, the lubrication properties of BP nanosheets as water-based and oil-based lubrication additives, and the friction and wear of BP/PVDF composites will be presented. Finally, the future challenges and opportunities in the use of BP materials as lubricants will be discussed. Keywords: black phosphorus; two-dimensional (2D) material; lubricant additive; self-lubricating composite materials; friction 1 Introduction certain period owing to the finite lubricant thickness, and it can be easily affected by the environment. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

Potential Ivvs.Gelbasedre
US010644304B2 ( 12 ) United States Patent ( 10 ) Patent No.: US 10,644,304 B2 Ein - Eli et al. (45 ) Date of Patent : May 5 , 2020 (54 ) METHOD FOR PASSIVE METAL (58 ) Field of Classification Search ACTIVATION AND USES THEREOF ??? C25D 5/54 ; C25D 3/665 ; C25D 5/34 ; ( 71) Applicant: Technion Research & Development HO1M 4/134 ; HOTM 4/366 ; HO1M 4/628 ; Foundation Limited , Haifa ( IL ) (Continued ) ( 72 ) Inventors : Yair Ein - Eli , Haifa ( IL ) ; Danny (56 ) References Cited Gelman , Haifa ( IL ) ; Boris Shvartsev , Haifa ( IL ) ; Alexander Kraytsberg , U.S. PATENT DOCUMENTS Yokneam ( IL ) 3,635,765 A 1/1972 Greenberg 3,650,834 A 3/1972 Buzzelli ( 73 ) Assignee : Technion Research & Development Foundation Limited , Haifa ( IL ) (Continued ) ( * ) Notice : Subject to any disclaimer , the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 CN 1408031 4/2003 U.S.C. 154 ( b ) by 56 days . EP 1983078 10/2008 (21 ) Appl. No .: 15 /300,359 ( Continued ) ( 22 ) PCT Filed : Mar. 31 , 2015 OTHER PUBLICATIONS (86 ) PCT No .: PCT/ IL2015 /050350 Hagiwara et al. in ( Acidic 1 - ethyl - 3 -methylimidazoliuum fluoride: a new room temperature ionic liquid in Journal of Fluorine Chem $ 371 (c ) ( 1 ), istry vol . 99 ( 1999 ) p . 1-3 ; ( Year: 1999 ). * (2 ) Date : Sep. 29 , 2016 (Continued ) (87 ) PCT Pub . No .: WO2015 / 151099 Primary Examiner — Jonathan G Jelsma PCT Pub . Date : Oct. 8 , 2015 Assistant Examiner Omar M Kekia (65 ) Prior Publication Data (57 ) ABSTRACT US 2017/0179464 A1 Jun . 22 , 2017 Disclosed is a method for activating a surface of metals , Related U.S. Application Data such as self- passivated metals , and of metal -oxide dissolu tion , effected using a fluoroanion -containing composition . -

Chemistry of the Noble Gases*
CHEMISTRY OF THE NOBLE GASES* By Professor K. K. GREE~woon , :.\I.Sc., sc.D .. r".lU.C. University of N ewca.stle 1tpon Tyne The inert gases, or noble gases as they are elements were unsuccessful, and for over now more appropriately called, are a remark 60 years they epitomized chemical inertness. able group of elements. The lightest, helium, Indeed, their electron configuration, s2p6, was recognized in the gases of the sun before became known as 'the stable octet,' and this it was isolated on ea.rth as its name (i]A.tos) fotmed the basis of the fit·st electronic theory implies. The first inert gas was isolated in of valency in 1916. Despite this, many 1895 by Ramsay and Rayleigh; it was named people felt that it should be possible to induce argon (apy6s, inert) and occurs to the extent the inert gases to form compounds, and many of 0·93% in the earth's atmosphere. The of the early experiments directed to this end other gases were all isolated before the turn have recently been reviewed.l of the century and were named neon (v€ov, There were several reasons why chemists new), krypton (KpVn'TOV, hidden), xenon believed that the inert gases might form ~€vov, stmnger) and radon (radioactive chemical compounds under the correct con emanation). Though they occur much less ditions. For example, the ionization poten abundantly than argon they cannot strictly tial of xenon is actually lower than those of be called rare gases; this can be illustrated hydrogen, nitrogen, oxygen, fl uorine and by calculating the volumes occupied a.t s.t.p. -
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
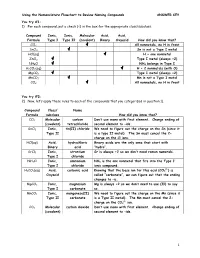
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes. -

Download (4Mb)
Original citation: Zhenqing, Li, He, Chaoyu, Ouyang, Tao, Zhang, Chunxiao, Tang, Chao, Roemer, Rudolf A. and Zhong, Jianxin (2018) New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity. Physical Review Applied, 9 . 044032. doi:10.1103/PhysRevApplied.9.044032 Permanent WRAP URL: http://wrap.warwick.ac.uk/101768 Copyright and reuse: The Warwick Research Archive Portal (WRAP) makes this work by researchers of the University of Warwick available open access under the following conditions. Copyright © and all moral rights to the version of the paper presented here belong to the individual author(s) and/or other copyright owners. To the extent reasonable and practicable the material made available in WRAP has been checked for eligibility before being made available. Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. Publisher statement: © 2018 American Physical Society Published version: https://doi.org/10.1103/PhysRevApplied.9.044032 A note on versions: The version presented here may differ from the published version or, version of record, if you wish to cite this item you are advised to consult the publisher’s version. Please see the ‘permanent WRAP url’ above for details on accessing the published version and note that access may require a subscription. For more information, please contact the WRAP Team at: [email protected] warwick.ac.uk/lib-publications New allotropes of phosphorene with remarkable stability and intrinsic piezoelectricity Zhenqing Li,1, 2 Chaoyu He,1, 2, ∗ Tao Ouyang,1, 2 Chunxiao Zhang,1, 2 Chao Tang,1, 2, y Rudolf A. -

Recent Advances in Synthesis, Properties, and Applications of Phosphorene
www.nature.com/npj2dmaterials REVIEW ARTICLE OPEN Recent advances in synthesis, properties, and applications of phosphorene Meysam Akhtar1,2, George Anderson1, Rong Zhao1, Adel Alruqi1, Joanna E. Mroczkowska3, Gamini Sumanasekera1,2 and Jacek B. Jasinski2 Since its first fabrication by exfoliation in 2014, phosphorene has been the focus of rapidly expanding research activities. The number of phosphorene publications has been increasing at a rate exceeding that of other two-dimensional materials. This tremendous level of excitement arises from the unique properties of phosphorene, including its puckered layer structure. With its widely tunable band gap, strong in-plane anisotropy, and high carrier mobility, phosphorene is at the center of numerous fundamental studies and applications spanning from electronic, optoelectronic, and spintronic devices to sensors, actuators, and thermoelectrics to energy conversion, and storage devices. Here, we review the most significant recent studies in the field of phosphorene research and technology. Our focus is on the synthesis and layer number determination, anisotropic properties, tuning of the band gap and related properties, strain engineering, and applications in electronics, thermoelectrics, and energy storage. The current needs and likely future research directions for phosphorene are also discussed. npj 2D Materials and Applications (2017) 1:5 ; doi:10.1038/s41699-017-0007-5 INTRODUCTION properties, including high carrier mobility, ultrahigh surface area, Since the discovery of graphene in 2004 (ref. 1), there has been a excellent thermal conductivity, and quantum confinement effect 9 quest for new two-dimensional (2D) materials aimed at fully have been well documented. However, the lack of band gap is a exploring new fundamental phenomena stemming from quantum serious limitation for the use of graphene in electronic devices. -
Curricular Axis Standard Competencies
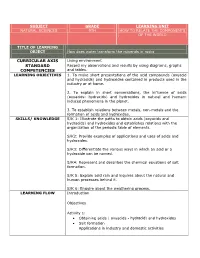
SUBJECT GRADE LEARNING UNIT NATURAL SCIENCES 9TH HOW TO RELATE THE COMPONENTS OF THE WORLD TITLE OF LEARNING OBJECT How does water transform the minerals in rocks CURRICULAR AXIS Living environment STANDARD Record my observations and results by using diagrams, graphs COMPETENCIES and tables. LEARNING OBJECTIVES 1. To make short presentations of the acid compounds (oxyacid and hydracids) and hydroxides contained in products used in the industry or at home. 2. To explain in short conversations, the influence of acids (oxyacids- hydracids) and hydroxides in natural and human- induced phenomena in the planet. 3. To establish relations between metals, non-metals and the formation of acids and hydroxides. SKILLS/ KNOWLEDGE S/K 1: Illustrate the paths to obtain acids (oxyacids and hydracids) and hydroxides and establishes relations with the organization of the periodic table of elements. S/K2: Provide examples of applications and uses of acids and hydroxides. S/K3: Differentiate the various ways in which an acid or a hydroxide can be named. S/K4: Represent and describes the chemical equations of salt formation. S/K 5: Explain acid rain and inquires about the natural and human processes behind it. S/K 6: Enquire about the weathering process. LEARNING FLOW Introduction Objectives Activity 1: Obtaining acids ( oxyacids - hydracid) and hydroxides Salt formation Applications in industry and domestic activities Activity 2: Nomenclature of acids (oxyacids - hydracid), hydroxides and salt. Activity 3: •Acid rain • Weathering Abstract Homework Evaluation Glossary ASSESSMENT This learning object is intended for the student to find a simple, GUIDELINE didactic and contextual guide. Likewise, students must develop the guide completely, including the different activities of this LO, by using the diverse tools and autonomous work suggested here.