3 • Molecules and Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential Ivvs.Gelbasedre
US010644304B2 ( 12 ) United States Patent ( 10 ) Patent No.: US 10,644,304 B2 Ein - Eli et al. (45 ) Date of Patent : May 5 , 2020 (54 ) METHOD FOR PASSIVE METAL (58 ) Field of Classification Search ACTIVATION AND USES THEREOF ??? C25D 5/54 ; C25D 3/665 ; C25D 5/34 ; ( 71) Applicant: Technion Research & Development HO1M 4/134 ; HOTM 4/366 ; HO1M 4/628 ; Foundation Limited , Haifa ( IL ) (Continued ) ( 72 ) Inventors : Yair Ein - Eli , Haifa ( IL ) ; Danny (56 ) References Cited Gelman , Haifa ( IL ) ; Boris Shvartsev , Haifa ( IL ) ; Alexander Kraytsberg , U.S. PATENT DOCUMENTS Yokneam ( IL ) 3,635,765 A 1/1972 Greenberg 3,650,834 A 3/1972 Buzzelli ( 73 ) Assignee : Technion Research & Development Foundation Limited , Haifa ( IL ) (Continued ) ( * ) Notice : Subject to any disclaimer , the term of this FOREIGN PATENT DOCUMENTS patent is extended or adjusted under 35 CN 1408031 4/2003 U.S.C. 154 ( b ) by 56 days . EP 1983078 10/2008 (21 ) Appl. No .: 15 /300,359 ( Continued ) ( 22 ) PCT Filed : Mar. 31 , 2015 OTHER PUBLICATIONS (86 ) PCT No .: PCT/ IL2015 /050350 Hagiwara et al. in ( Acidic 1 - ethyl - 3 -methylimidazoliuum fluoride: a new room temperature ionic liquid in Journal of Fluorine Chem $ 371 (c ) ( 1 ), istry vol . 99 ( 1999 ) p . 1-3 ; ( Year: 1999 ). * (2 ) Date : Sep. 29 , 2016 (Continued ) (87 ) PCT Pub . No .: WO2015 / 151099 Primary Examiner — Jonathan G Jelsma PCT Pub . Date : Oct. 8 , 2015 Assistant Examiner Omar M Kekia (65 ) Prior Publication Data (57 ) ABSTRACT US 2017/0179464 A1 Jun . 22 , 2017 Disclosed is a method for activating a surface of metals , Related U.S. Application Data such as self- passivated metals , and of metal -oxide dissolu tion , effected using a fluoroanion -containing composition . -

Chemistry of the Noble Gases*
CHEMISTRY OF THE NOBLE GASES* By Professor K. K. GREE~woon , :.\I.Sc., sc.D .. r".lU.C. University of N ewca.stle 1tpon Tyne The inert gases, or noble gases as they are elements were unsuccessful, and for over now more appropriately called, are a remark 60 years they epitomized chemical inertness. able group of elements. The lightest, helium, Indeed, their electron configuration, s2p6, was recognized in the gases of the sun before became known as 'the stable octet,' and this it was isolated on ea.rth as its name (i]A.tos) fotmed the basis of the fit·st electronic theory implies. The first inert gas was isolated in of valency in 1916. Despite this, many 1895 by Ramsay and Rayleigh; it was named people felt that it should be possible to induce argon (apy6s, inert) and occurs to the extent the inert gases to form compounds, and many of 0·93% in the earth's atmosphere. The of the early experiments directed to this end other gases were all isolated before the turn have recently been reviewed.l of the century and were named neon (v€ov, There were several reasons why chemists new), krypton (KpVn'TOV, hidden), xenon believed that the inert gases might form ~€vov, stmnger) and radon (radioactive chemical compounds under the correct con emanation). Though they occur much less ditions. For example, the ionization poten abundantly than argon they cannot strictly tial of xenon is actually lower than those of be called rare gases; this can be illustrated hydrogen, nitrogen, oxygen, fl uorine and by calculating the volumes occupied a.t s.t.p. -

Midas Arsine
® Midas SENSOR CARTRIDGE SPECIFICATIONS Arsine (AsH3), Germane (GeH4) MIDAS-S-ASH, MIDAS-E-ASH Gas Measured Measured Arsine (AsH3) Cross Sensitivities Each Midas® sensor is potentially cross sensitive to other gases Cartridge Part Number MIDAS-S-ASH 1 year standard warranty MIDAS-E-ASH 2 year extended warranty and this may cause a gas reading when exposed to other gases than those originally designated. The table below presents typical Sensor Technology 3 electrode electrochemical cell readings that will be observed when a new sensor cartridge is Measuring Range (ppm) AsH 0 – 0.2ppm exposed to the cross sensitive gas (or a mixture of gases containing 3 the cross sensitive species). Minimum Alarm 1 Set Point 0.025ppm Gas / Vapor Chemical Concentration Reading Repeatability < ± 2% of measured value Formula applied (ppm) (ppm AsH3) Linearity < ± 10% of measured value Ammonia NH3 108 <0.1 Response Time t62.5 < 15 seconds Carbon Dioxide CO2 5,000 0 Sensor Cartridge Life Expectancy ≥ 24 months under typical application conditions Carbon Monoxide CO 85 0 Operating Temperature 0°C to + 40°C (32°C to 104°F) Chlorine CI2 TBD <-0.05 Effect of Temperature Diborane B2H6 0.1 0.05 Zero < ± 0.004 ppm / °C (0° to 40°C) Sensitivity < ± 0.9% of measured value / °C Disilane Si2H6 0.27 0.12 Operating Humidity (continuous) 10 – 95% rH non-condensing Germane GeH4 0.27 0.05 Effect of Humidity Hydrogen H2 3100 <-0.05 Zero Initial short term drift at abrupt rH change (< 0.001 ppm / % rH) Sensitivity < ± 0.2% of measured value / % rH Hydrogen Chloride HCI 7.9 0 -

Flow Injection Electrochemical Hydride Generation of Hydrogen Selenide on Lead Cathode: Critical Study of the Influence of Experimental Parameters
ANALYTICAL SCIENCES MARCH 2003, VOL. 19 367 2003 © The Japan Society for Analytical Chemistry Flow Injection Electrochemical Hydride Generation of Hydrogen Selenide on Lead Cathode: Critical Study of the Influence of Experimental Parameters Eduardo BOLEA, Francisco LABORDA,† and Juan R. CASTILLO Analytical Spectroscopy and Sensors Group, Department of Analytical Chemistry, University of Zaragoza, Zaragoza, Spain A flow injection system for the determination of selenium by electrochemical hydride generation and quartz tube atomic absorption spectrometry is described. The generator consists of an electrolytic flow-through cell with a concentric arrangement and a packed cathode made of particulated lead. The influences of sample flow rate, carrier gas flow rate and electrolysis current on the hydrogen selenide generation have been critically studied. Both sample flow rate and electrolysis current play important roles in the efficiency of the hydride generation process. A characteristic mass of 2.4 ng and a concentration detection limit of 17 µg l–1 were obtained for a sample volume of 420 µl. (Received January 22, 2002; Accepted September 25, 2002) The electrochemical generation of different hydrides on lead Introduction cathodes, the material that offers the highest hydrogen overpotential among those commonly used, has been studied by The formation of volatile covalent hydrides of a number of several authors.3–5,7–11 Sand3 and Thompson4,5 obtained elements (As, Bi, Ge, Sn, Sb, Se, Te and Pb) by reaction with generation efficiencies of 100%, whereas efficiencies of 86,11 sodium tetrahydroborate in acidic media has been widely used 928 and 60%11 were obtained for arsine, stibine and hydrogen for sample introduction in atomic spectrometry. -

Biological Chemistry of Hydrogen Selenide
antioxidants Review Biological Chemistry of Hydrogen Selenide Kellye A. Cupp-Sutton † and Michael T. Ashby *,† Department of Chemistry and Biochemistry, University of Oklahoma, Norman, OK 73019, USA; [email protected] * Correspondence: [email protected]; Tel.: +1-405-325-2924 † These authors contributed equally to this work. Academic Editors: Claus Jacob and Gregory Ian Giles Received: 18 October 2016; Accepted: 8 November 2016; Published: 22 November 2016 Abstract: There are no two main-group elements that exhibit more similar physical and chemical properties than sulfur and selenium. Nonetheless, Nature has deemed both essential for life and has found a way to exploit the subtle unique properties of selenium to include it in biochemistry despite its congener sulfur being 10,000 times more abundant. Selenium is more easily oxidized and it is kinetically more labile, so all selenium compounds could be considered to be “Reactive Selenium Compounds” relative to their sulfur analogues. What is furthermore remarkable is that one of the most reactive forms of selenium, hydrogen selenide (HSe− at physiologic pH), is proposed to be the starting point for the biosynthesis of selenium-containing molecules. This review contrasts the chemical properties of sulfur and selenium and critically assesses the role of hydrogen selenide in biological chemistry. Keywords: biological reactive selenium species; hydrogen selenide; selenocysteine; selenomethionine; selenosugars; selenophosphate; selenocyanate; selenophosphate synthetase thioredoxin reductase 1. Overview of Chalcogens in Biology Chalcogens are the chemical elements in group 16 of the periodic table. This group, which is also known as the oxygen family, consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive element polonium (Po). -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

2020 Emergency Response Guidebook
2020 A guidebook intended for use by first responders A guidebook intended for use by first responders during the initial phase of a transportation incident during the initial phase of a transportation incident involving hazardous materials/dangerous goods involving hazardous materials/dangerous goods EMERGENCY RESPONSE GUIDEBOOK THIS DOCUMENT SHOULD NOT BE USED TO DETERMINE COMPLIANCE WITH THE HAZARDOUS MATERIALS/ DANGEROUS GOODS REGULATIONS OR 2020 TO CREATE WORKER SAFETY DOCUMENTS EMERGENCY RESPONSE FOR SPECIFIC CHEMICALS GUIDEBOOK NOT FOR SALE This document is intended for distribution free of charge to Public Safety Organizations by the US Department of Transportation and Transport Canada. This copy may not be resold by commercial distributors. https://www.phmsa.dot.gov/hazmat https://www.tc.gc.ca/TDG http://www.sct.gob.mx SHIPPING PAPERS (DOCUMENTS) 24-HOUR EMERGENCY RESPONSE TELEPHONE NUMBERS For the purpose of this guidebook, shipping documents and shipping papers are synonymous. CANADA Shipping papers provide vital information regarding the hazardous materials/dangerous goods to 1. CANUTEC initiate protective actions. A consolidated version of the information found on shipping papers may 1-888-CANUTEC (226-8832) or 613-996-6666 * be found as follows: *666 (STAR 666) cellular (in Canada only) • Road – kept in the cab of a motor vehicle • Rail – kept in possession of a crew member UNITED STATES • Aviation – kept in possession of the pilot or aircraft employees • Marine – kept in a holder on the bridge of a vessel 1. CHEMTREC 1-800-424-9300 Information provided: (in the U.S., Canada and the U.S. Virgin Islands) • 4-digit identification number, UN or NA (go to yellow pages) For calls originating elsewhere: 703-527-3887 * • Proper shipping name (go to blue pages) • Hazard class or division number of material 2. -

Xenon Iron Oxides Predicted As Potential Xe Hosts in Earth’S Lower Mantle
ARTICLE https://doi.org/10.1038/s41467-020-19107-y OPEN Xenon iron oxides predicted as potential Xe hosts in Earth’s lower mantle ✉ Feng Peng 1,2,8, Xianqi Song 3,4,8, Chang Liu4,5,6, Quan Li 3,4,5,6 , Maosheng Miao 2, ✉ ✉ Changfeng Chen 7 & Yanming Ma 3,4,5 An enduring geological mystery concerns the missing xenon problem, referring to the abnormally low concentration of xenon compared to other noble gases in Earth’s atmosphere. 1234567890():,; Identifying mantle minerals that can capture and stabilize xenon has been a great challenge in materials physics and xenon chemistry. Here, using an advanced crystal structure search algorithm in conjunction with first-principles calculations we find reactions of xenon with recently discovered iron peroxide FeO2, forming robust xenon-iron oxides Xe2FeO2 and XeFe3O6 with significant Xe-O bonding in a wide range of pressure-temperature conditions corresponding to vast regions in Earth’s lower mantle. Calculated mass density and sound velocities validate Xe-Fe oxides as viable lower-mantle constituents. Meanwhile, Fe oxides do not react with Kr, Ar and Ne. It means that if Xe exists in the lower mantle at the same pressures as FeO2, xenon-iron oxides are predicted as potential Xe hosts in Earth’s lower mantle and could provide the repository for the atmosphere’s missing Xe. These findings establish robust materials basis, formation mechanism, and geological viability of these Xe-Fe oxides, which advance fundamental knowledge for understanding xenon chemistry and physics mechanisms for the possible deep-Earth Xe reservoir. 1 College of Physics and Electronic Information & Henan Key Laboratory of Electromagnetic Transformation and Detection, Luoyang Normal University, 471022 Luoyang, China. -

Properties of Acids
Properties of Acids Solutions of acids have a sour taste Don’t taste them in the lab !!! 1 They change the colors of many indicators Acids turn blue litmus to red Acids turn bromothymol blue from blue to yellow They react with metals to generate hydrogen gas, H2 1 Metal Activity Series More active Li, K, Ca, Na, Mg, Al, Mn, Zn, Fe, Co, Ni, Pb, H, Cu, Hg, Ag, Pt, Au Less active Active enough to displace Cannot displace hydrogen from an acid hydrogen from an acid 2 Properties of Acids They react with metal oxides forming the salt of the metal and water CaO + 2HCl → CaCl2 + H2O They react with metal hydroxides forming the salt of the metal and water Ca(OH)2 + 2HCl → CaCl2 + 2H2O 3 Oxides Compounds of oxygen and another element There are two ways to name oxides Based on the oxidation number of the element Li2O – lithium oxide BaO – barium oxide FeO – iron(II) oxide Fe2O3 – iron(III) oxide Based on the number of atoms of each element Li2O – dilithium oxide BaO – barium oxide FeO – iron oxide Fe2O3 – diiron trioxide 4 Example 1 Name the following compounds: BeO, Al2O3, Cu2O, OsO4, Cr2O3, CrO3 5 Example 2 Write formulas for the following compounds: Potassium oxide Boron oxide Diindium trioxide Cobalt(II) oxide Dinitrogen pentoxide Rhenium(VI) oxide Xenon tetroxide Carbon monoxide Carbon dioxide Manganese(VII) oxide 6 Example 3 Write total and net ionic equations for the reaction between cobalt (III) oxide and diluted hydroiodic acid 7 Example 4 Write total and net ionic equations for the reaction between dialuminum -

Coordination Chemistry of Xenon
Coordination Chemistry of Xenon Paul Griffin Literature Seminar 11/14/19 Situated on the far side of the periodic table, the chemical inertness of the noble gases has led to their widespread applications ranging from gas-discharge lamps and ion engines for space travel to medical applications including as MRI contrast agents and radiotherapy.1 Though all practical applications of the noble gases center around Xe (0), understanding the reactivity and bonding of xenon in higher oxidation states is fundamental to investigating chemistry at the far end of the periodic table. The reactivity of the noble gases, in particular xenon, was first uncovered when Bartlett, who had previously prepared dioxygenyl hexafluoroplatinate (V) (O2PtF6), noted similar ionization energies for molecular oxygen and atomic xenon (a difference of 0.1 eV).2 Mixing of xenon with PtF6 vapor led to the formation of an orange-yellow solid led to the synthesis of the first noble gas compound, xenon hexafluoroplatinate (V) (XePtF6). The chemistry of xenon has evolved significantly since its’ discovery to include an assortment of xenon fluorides, oxides, oxylfluorides, organoxenon3, and metalloxenon compounds such as the tetraxenoaurate (II) cation (Figure 1).4 These species have weak covalent bonding between Xe and the oxygen/fluorine moieties.5 The chemistry of xenon is dominated by the 2+, 4+, and 6+ oxidation states, though Xe (VIII) compounds are known in the form of perxenates and xenon tetroxide. These primarily ionic species, particularly the oxides, oxylfluorides, and fluorides, serve as precursors to noncovalent adducts of xenon. Figure 1. Organoxenon and metalloxenon species Recent work has led to the preparation of xenon adducts with noncovalently interacting ligands such as acetonitrile6a, 6b, ketones6c, sulfoxides6c, and crown ethers6d (Figure 2) capable of weakly coordinating to xenon salts to afford stable species at room temperature. -
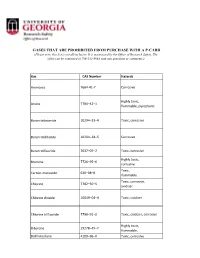
Lists of Gases for Pcard Manual.Xlsx
GASES THAT ARE PROHIBITED FROM PURCHASE WITH A P-CARD (Please note, this list is not all-inclusive. It is maintained by the Office of Research Safety. The office can be contacted at 706-542-9088 with any questions or comments.) Gas CAS Number Hazards Ammonia 7664‐41‐7 Corrosive Highly toxic, Arsine 7784–42–1 flammable, pyrophoric Boron tribromide 10294–33–4 Toxic, corrosive Boron trichloride 10294–34–5 Corrosive Boron trifluoride 7637–07–2 Toxic, corrosive Highly toxic, Bromine 7726–95–6 corrosive Toxic, Carbon monoxide 630–08–0 flammable Toxic, corrosive, Chlorine 7782–50–5 oxidizer Chlorine dioxide 10049–04–4 Toxic, oxidizer Chlorine trifluoride 7790–91–2 Toxic, oxidizer, corrosive Highly toxic, Diborane 19278–45–7 flammable, Dichlorosilane 4109–96–0 Toxic, corrosive Ethylene oxide 75–21–8 Toxic, flammable Highly toxic, corrosive, Fluorine 7782–41–4 oxidizer Highly toxic, Germane 7782–65–2 flammable Hydrogen bromide 10035–10–6 Toxic, corrosive Hydrogen chloride 7647–01–0 Toxic, corrosive Hydrogen cyanide 74–90–8 Highly toxic, flammable Hydrogen fluoride 7664–39–3 Toxic, corrosive Hydrogen iodide 10034‐85‐2 Toxic, corrosive Highly toxic, Hydrogen selenide 7783–07–5 flammable Toxic, flammable, Hydrogen sulfide 7783–06–4 corrosive Methyl bromide 74–83–9 Toxic Methyl isocyanate 624‐83‐9 Highly toxic, flammable Methyl mercaptan 74–93–1 Toxic, flammable Nickel carbonyl 13463–39–3 Highly toxic, flammable Highly toxic, Nitric oxide 10102–43–9 oxidizer Highly toxic, Nitrogen dioxide 10102–44–0 oxidizer, corrosive Highly toxic, Ozone 10028‐15‐6