Sodium Chlorite Treatment of Cooling Water with Chlorine Dioxide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Decontamination of Indoor and Outdoor Materials with Aqueous Chlorine Dioxide Solutions
EPA 600/R-12/516 | May 2012 | www.epa.gov/ord Decontamination of Indoor and Outdoor Materials with Aqueous Chlorine Dioxide Solutions Office of Research and Development National Homeland Security Research Center EPA/600/R/12/516 May 2012 Decontamination of Indoor and Outdoor Materials with Aqueous Chlorine Dioxide Solutions U.S. Environmental Protection Agency Research Triangle Park, NC 27711 ii Disclaimer The U.S. Environmental Protection Agency (EPA), through its Office of Research and Development’s (ORD) National Homeland Security Research Center (NHSRC), funded, directed and managed this work through Contract Number EP-C-10-001 with Battelle. This report has been peer and administratively reviewed and has been approved for publication as an EPA document. Mention of trade names or commercial products does not constitute endorsement or recommendation for use of a specific product. Questions concerning this document or its application should be addressed to: Joseph Wood National Homeland Security Research Center Office of Research and Development U.S. Environmental Protection Agency Mail Code E343-06 Research Triangle Park, NC 27711 919-541-5029 iii Foreword Following the events of September 11, 2001, addressing the critical needs related to homeland security became a clear requirement with respect to EPA’s mission to protect human health and the environment. Presidential Directives further emphasized EPA as the primary federal agency responsible for the country’s water supplies and for decontamination following a chemical, biological, and/or radiological (CBR) attack. To support EPA’s mission to assist in and lead response and recovery activities associated with CBR incidents of national significance, the National Homeland Security Research Center (NHSRC) was established to conduct research and deliver products that improve the capability of the Agency and other federal, state, and local agencies to carry out their homeland security responsibilities. -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Crops 2019 Sunset Reviews: §§205.601, 205.602 (Pdf)
Sunset 2019 Meeting 2 - Review Crops Substances November 2017 Note: With the exception of biodegradable biobased mulch film, the materials included in this list are undergoing early sunset review as part of November 18, 2016 NOSB recommendation on efficient workload re-organization. As part of the National List sunset review process, the NOSB Crops Subcommittee has evaluated the need for the continued allowance for or prohibition of the following substances for use in organic crop production. Reference: 7 CFR 205.601 Synthetic substances allowed for use in organic crop production. Chlorine materials: calcium hypochlorite, chlorine dioxide, sodium hypochlorite Herbicides, soap-based Biodegradable biobased mulch film Boric acid Sticky traps/barriers Coppers, fixed Copper sulfate Humic acids Micronutrients: soluble boron products Micronutrients: sulfates, carbonates, oxides, or silicates of zinc, copper, iron, manganese, molybdenum, selenium, and cobalt Vitamins B1, C, E 205.602 Nonsynthetic substances prohibited for use in organic crop production Lead salts Tobacco dust (nicotine sulfate) NOSB October 2017 proposals and discussion documents 17/175 Chlorine materials - Calcium Hypochlorite Reference: 205.601(a) - As algicide, disinfectants, and sanitizer, including irrigation system cleaning systems. (2) Chlorine materials -For pre-harvest use, residual chlorine levels in the water in direct crop contact or as water from cleaning irrigation systems applied to soil must not exceed the maximum residual disinfectant limit under the Safe Drinking -
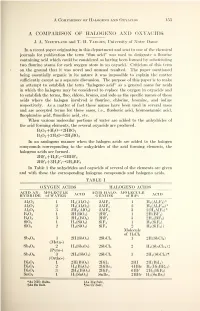
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

United States Patent Office Patented Feb
2,701,781 United States Patent Office Patented Feb. 8, 1955 2 pound in the presence of an excess of water and allow ing the two to combine. The chlorine dioxide can be 2,701,781 generated in situ or externally of the solution or suspen AQUEOUS CHLORINE DIOXIDE ANTISEPTIC sion of the boron compound, as will become more clear COMPOSITIONS AND PRODUCTIONTHEREOF from the discussion given below. Moises L. de Guevara, Mexico City, Mexico, assignor, by The following examples, in which all parts indicated are mesne assignments, of fifty-seven and one-half per cent by Weight, of actual operations in accordance with this to Charles Wan Buren, Washington, D. C., and forty invention, are given in order to provide an easy com two and one-half per cent to Pearlman, Baldridge, prehension of the type of materials and procedures with Lyons and Browning, Washington, D. C. which the invention is concerned. Example I No Drawing. Application December 30, 1949, An antiseptic composition is produced from the follow Serial No. 136,149 ing ingredients: 9 Claims. (C. 167-17) 15 Parts Sodium tetraborate---------------------------- 20 This invention relates to new antiseptic compositions Boric acid------------------------------------ 20 having great antiseptic strength. It is also concerned Sodium perborate----------------------------- 3 with methods for the production of these new products. Potassium chlorate---------------------------- 2 Innumerable materials have been tested and experi 20 Sulfuric acid---------------------------------- 1. mented with in order to uncover successful antiseptics Hydrochloric acid----------------------------- 1. for general application. Notwithstanding the large Para amino benzoic acid------------------------ .1 amount of investigation made in this field, a really Sat Pye ---------------------------------------- 0.1 isfactory, universal antiseptic has not been provided here Water in an amount to make 1000 parts. -
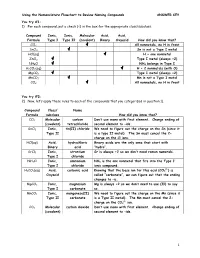
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes. -

Chlorine Dioxide
Common Name: CHLORINE DIOXIDE CAS Number: 10049-04-4 DOT Number: NA 9191 (Hydrate, frozen) DOT Hazard Class: FORBIDDEN (Not Hydrate) RTK Substance number: 0368 5.1 (Oxidizer) Date: June 1998 Revision: December 2005 ------------------------------------------------------------------------- ------------------------------------------------------------------------- HAZARD SUMMARY * Chlorine Dioxide can affect you when breathed in. * Exposure to hazardous substances should be routinely * Contact can irritate the skin and eyes causing watery eyes evaluated. This may include collecting personal and area and seeing halos around the lights. air samples. You can obtain copies of sampling results * Breathing Chlorine Dioxide can irritate the nose and from your employer. You have a legal right to this throat causing coughing and wheezing. information under the OSHA Access to Employee * Breathing Chlorine Dioxide can irritate the lungs causing Exposure and Medical Records Standard (29 CFR coughing and/or shortness of breath. Higher exposures 1910.1020). can cause a build-up of fluid in the lungs (pulmonary * If you think you are experiencing any work-related health edema), a medical emergency, with severe shortness of problems, see a doctor trained to recognize occupational breath. diseases. Take this Fact Sheet with you. * Chlorine Dioxide is a HIGHLY FLAMMABLE and * ODOR THRESHOLD = 0.1 ppm. REACTIVE gas and a DANGEROUS FIRE and * The range of accepted odor threshold values is quite EXPLOSION HAZARD. broad. Caution should be used in relying on odor alone as * DOT regulations FORBID the transport of Chlorine a warning of potentially hazardous exposures. Dioxide in Not Hydrate form. WORKPLACE EXPOSURE LIMITS IDENTIFICATION OSHA: The legal airborne permissible exposure limit Chlorine Dioxide is a yellow to red gas with an irritating odor (PEL) is 0.1 ppm averaged over an 8-hour similar to Chlorine. -

Toxicological Profile for Chlorine Dioxide and Chlorite
TOXICOLOGICAL PROFILE FOR CHLORINE DIOXIDE AND CHLORITE U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Agency for Toxic Substances and Disease Registry September 2004 CHLORINE DIOXIDE AND CHLORITE ii DISCLAIMER The use of company or product name(s) is for identification only and does not imply endorsement by the Agency for Toxic Substances and Disease Registry. CHLORINE DIOXIDE AND CHLORITE iii UPDATE STATEMENT Toxicological Profile for Chlorine Dioxide and Chlorite, Draft for Public Comment was released in September 2002. This edition supersedes any previously released draft or final profile. Toxicological profiles are revised and republished as necessary. For information regarding the update status of previously released profiles, contact ATSDR at: Agency for Toxic Substances and Disease Registry Division of Toxicology/Toxicology Information Branch 1600 Clifton Road NE, Mailstop F-32 Atlanta, Georgia 30333 CHLORINE DIOXIDE AND CHLORITE vi *Legislative Background The toxicological profiles are developed in response to the Superfund Amendments and Reauthorization Act (SARA) of 1986 (Public law 99-499) which amended the Comprehensive Environmental Response, Compensation, and Liability Act of 1980 (CERCLA or Superfund). This public law directed ATSDR to prepare toxicological profiles for hazardous substances most commonly found at facilities on the CERCLA National Priorities List and that pose the most significant potential threat to human health, as determined by ATSDR and the EPA. The availability of the revised priority list of 275 hazardous substances was announced in the Federal Register on November 17, 1997 (62 FR 61332). For prior versions of the list of substances, see Federal Register notices dated April 29, 1996 (61 FR 18744); April 17, 1987 (52 FR 12866); October 20, 1988 (53 FR 41280); October 26, 1989 (54 FR 43619); October 17, 1990 (55 FR 42067); October 17, 1991 (56 FR 52166); October 28, 1992 (57 FR 48801); and February 28, 1994 (59 FR 9486). -
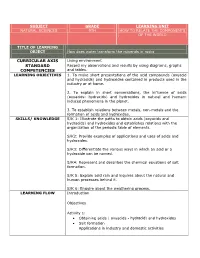
Curricular Axis Standard Competencies
SUBJECT GRADE LEARNING UNIT NATURAL SCIENCES 9TH HOW TO RELATE THE COMPONENTS OF THE WORLD TITLE OF LEARNING OBJECT How does water transform the minerals in rocks CURRICULAR AXIS Living environment STANDARD Record my observations and results by using diagrams, graphs COMPETENCIES and tables. LEARNING OBJECTIVES 1. To make short presentations of the acid compounds (oxyacid and hydracids) and hydroxides contained in products used in the industry or at home. 2. To explain in short conversations, the influence of acids (oxyacids- hydracids) and hydroxides in natural and human- induced phenomena in the planet. 3. To establish relations between metals, non-metals and the formation of acids and hydroxides. SKILLS/ KNOWLEDGE S/K 1: Illustrate the paths to obtain acids (oxyacids and hydracids) and hydroxides and establishes relations with the organization of the periodic table of elements. S/K2: Provide examples of applications and uses of acids and hydroxides. S/K3: Differentiate the various ways in which an acid or a hydroxide can be named. S/K4: Represent and describes the chemical equations of salt formation. S/K 5: Explain acid rain and inquires about the natural and human processes behind it. S/K 6: Enquire about the weathering process. LEARNING FLOW Introduction Objectives Activity 1: Obtaining acids ( oxyacids - hydracid) and hydroxides Salt formation Applications in industry and domestic activities Activity 2: Nomenclature of acids (oxyacids - hydracid), hydroxides and salt. Activity 3: •Acid rain • Weathering Abstract Homework Evaluation Glossary ASSESSMENT This learning object is intended for the student to find a simple, GUIDELINE didactic and contextual guide. Likewise, students must develop the guide completely, including the different activities of this LO, by using the diverse tools and autonomous work suggested here.