Hypochlorous Acid Handling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ins and Outs of Dechlorination
The Ins and Outs of Dechlorination If you plan on using a municipal water supply for chlorine is added to water, it will first react with growing fish you will require a dechlorination step any reducing agents, including any ammonia that in your water treatment process. Most municipal may be present. Hypochlorous acid reacts with water systems use chlorine or chloramine (a com- ammonia to form chloramines as follows: bination of chlorine and ammonia) to render the water safe for human consumption. While rela- HOCL + NH3 _ H2O + NH2Cl (monochloramine) tively harmless to humans in minute amounts, HOCL + NH2Cl _ H2O + NHCl2 (dichloramine) chlorine can be deadly to fish. In order to under- HOCL + NHCl2 _ H2O + NCl3 (trichloramine) BY: CARLA MACQUARRIE stand how to dechlorinate water, there must first AND SEAN WILTON be an understanding of how and why chlorine is The formation of chloramines is also dependent used as a disinfecting agent. on pH and temperature, as well as on the concen- tration of ammonia. With the added presence of Chlorine is water soluble gas (7160 mg/L these chloramines, the available chlorine is at 20°C and 1atm) that hydrolyzes rapidly referred to as combined available chlorine (CAC). to form hypochlorous acid (HOCl). This When these reactions are complete, residual reaction forms the basis for the applica- chlorine will accumulate, either in the form of FAC tion of chlorine as a disinfectant and oxi- or CAC. Total residual chlorine refers to the sum of dant as follows: the free and combined forms of residual chlorine. As a disinfectant, the FAC component is more Cl2 + H20 —> HOCl + H+ + Cl- effective than the CAC – this is because chlo- ramines are considered to have only a moderate The by-product, hypochlorous acid(HOCl) biocidal activity against bacteria and a low bioci- ionizes to produce the hypochlorite (OCl-) dal activity against viruses and cysts. -

United States Patent Office
2,752,270 United States Patent Office Patented June 26, 1956 2 These impurities impair the crystallization of the solu 2,752,270 tions for the recovery of glucose; on the one hand, they PROCESS OF HYDROLYZNG wooD N PRE. increase the solubility of the glucose in the solvent, and PARNG CRYSTALLINE GLUCOSE on the other hand they retard the growth of the glucose Hugo Specht, Mannheim-Rheinau, Germany, assignor 5 crystals which remain so snail as to be very difficult to to Deutsche Bergin-Aktiengesellschaft, Mannheim separate from the syrup. I have found that crystalline Rheinau, Germany glucose can be obtained in good yields only when the No Drawing. Application January 5, 1954, Wood sugar Solution subjected to crystallization is so Serial No. 402,401 prepared as to consist essentially of glucose and to be free Claims priority, application Germany January 31, 1949 0 as far as possible from the recited impurities, including mannose. According to the invention, cellulosic ma 4 Claims. (C. 127-37) terial, for instance wood, is essentially freed from hemi The invention relates to improvements in the prepara celluloses; the residue should contain not more than tion of crystalline glucose by the hydrolysis of wood and about 5-6 per cent of sugars other than glucose, cal is a continuation-in-part of my copending application, 5 culated on the total content of carbohydrates, and is Serial No. 242,172, filed August 16, 1951, now aban then hydrolyzed with superconcentrated (39–42%) hy doned. drochloric acid. The thus obtained strongly acidic sugar Several processes have already been proposed for the solution is freed from hydrochloric acid by evaporation recovery of crystalline glucose from wood sugar solu So as to leave only a small residue of the acid; the mass tions, but they could not be economically operated on a 20 is then diluted to form a liquor containing about 10 to large scale. -
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Guidance for Identification and Naming of Substance Under REACH
Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 GUIDANCE Guidance for identification and naming of substances under REACH and CLP May 2017 Version 2.1 2 Guidance for identification and naming of substances under REACH and CLP Version 2.1 - May 2017 LEGAL NOTICE This document aims to assist users in complying with their obligations under the REACH and CLP regulations. However, users are reminded that the text of the REACH and CLP Regulations is the only authentic legal reference and that the information in this document does not constitute legal advice. Usage of the information remains under the sole responsibility of the user. The European Chemicals Agency does not accept any liability with regard to the use that may be made of the information contained in this document. Guidance for identification and naming of substances under REACH and CLP Reference: ECHA-16-B-37.1-EN Cat. Number: ED-07-18-147-EN-N ISBN: 978-92-9495-711-5 DOI: 10.2823/538683 Publ.date: May 2017 Language: EN © European Chemicals Agency, 2017 If you have any comments in relation to this document please send them (indicating the document reference, issue date, chapter and/or page of the document to which your comment refers) using the Guidance feedback form. The feedback form can be accessed via the EVHA Guidance website or directly via the following link: https://comments.echa.europa.eu/comments_cms/FeedbackGuidance.aspx European Chemicals Agency Mailing address: P.O. Box 400, FI-00121 Helsinki, Finland Visiting address: Annankatu 18, Helsinki, Finland Guidance for identification and naming of substances under 3 REACH and CLP Version 2.1 - May 2017 PREFACE This document describes how to name and identify a substance under REACH and CLP. -

Warning: the Following Lecture Contains Graphic Images
What the новичок (Novichok)? Why Chemical Warfare Agents Are More Relevant Than Ever Matt Sztajnkrycer, MD PHD Professor of Emergency Medicine, Mayo Clinic Medical Toxicologist, Minnesota Poison Control System Medical Director, RFD Chemical Assessment Team @NoobieMatt #ITLS2018 Disclosures In accordance with the Accreditation Council for Continuing Medical Education (ACCME) Standards, the American Nurses Credentialing Center’s Commission (ANCC) and the Commission on Accreditation for Pre-Hospital Continuing Education (CAPCE), states presenters must disclose the existence of significant financial interests in or relationships with manufacturers or commercial products that may have a direct interest in the subject matter of the presentation, and relationships with the commercial supporter of this CME activity. The presenter does not consider that it will influence their presentation. Dr. Sztajnkrycer does not have a significant financial relationship to report. Dr. Sztajnkrycer is on the Editorial Board of International Trauma Life Support. Specific CW Agents Classes of Chemical Agents: The Big 5 The “A” List Pulmonary Agents Phosgene Oxime, Chlorine Vesicants Mustard, Phosgene Blood Agents CN Nerve Agents G, V, Novel, T Incapacitating Agents Thinking Outside the Box - An Abbreviated List Ammonia Fluorine Chlorine Acrylonitrile Hydrogen Sulfide Phosphine Methyl Isocyanate Dibotane Hydrogen Selenide Allyl Alcohol Sulfur Dioxide TDI Acrolein Nitric Acid Arsine Hydrazine Compound 1080/1081 Nitrogen Dioxide Tetramine (TETS) Ethylene Oxide Chlorine Leaks Phosphine Chlorine Common Toxic Industrial Chemical (“TIC”). Why use it in war/terror? Chlorine Density of 3.21 g/L. Heavier than air (1.28 g/L) sinks. Concentrates in low-lying areas. Like basements and underground bunkers. Reacts with water: Hypochlorous acid (HClO) Hydrochloric acid (HCl). -

Dissociation Constants and Ph-Titration Curves at Constant Ionic Strength from Electrometric Titrations in Cells Without Liquid
U. S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS RESEARCH PAPER RP1537 Part of Journal of Research of the N.ational Bureau of Standards, Volume 30, May 1943 DISSOCIATION CONSTANTS AND pH-TITRATION CURVES AT CONSTANT IONIC STRENGTH FROM ELECTRO METRIC TITRATIONS IN CELLS WITHOUT LIQUID JUNCTION : TITRATIONS OF FORMIC ACID AND ACETIC ACID By Roger G. Bates, Gerda L. Siegel, and S. F. Acree ABSTRACT An improved method for obtaining the titration curves of monobasic acids is outlined. The sample, 0.005 mole of the sodium salt of the weak acid, is dissolver! in 100 ml of a 0.05-m solution of sodium chloride and titrated electrometrically with an acid-salt mixture in a hydrogen-silver-chloride cell without liquid junction. The acid-salt mixture has the composition: nitric acid, 0.1 m; pot assium nitrate, 0.05 m; sodium chloride, 0.05 m. The titration therefore is performed in a. medium of constant chloride concentration and of practically unchanging ionic strength (1'=0.1) . The calculations of pH values and of dissociation constants from the emf values are outlined. The tit ration curves and dissociation constants of formic acid and of acetic acid at 25 0 C were obtained by this method. The pK values (negative logarithms of the dissociation constants) were found to be 3.742 and 4. 754, respectively. CONTENTS Page I . Tntroduction __ _____ ~ __ _______ . ______ __ ______ ____ ________________ 347 II. Discussion of the titrat ion metbod __ __ ___ ______ _______ ______ ______ _ 348 1. Ti t;at~on. clU,:es at constant ionic strength from cells without ltqUld JunctlOlL - - - _ - __ _ - __ __ ____ ____ _____ __ _____ ____ __ _ 349 2. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

Active Chlorine Released from Hypochlorous Acid
Regulation (EU) No 528/2012 concerning the making available on the market and use of biocidal products Evaluation of active substances Assessment Report ★ ★ ★ ★ * ★ * ★★ Active chlorine released from hypochlorous acid Product-type 1 (Human hygiene) July 2020 Slovak Republic Active chlorine released from Product-type 1 July 2020 hypochlorous acid CONTENTS 1. STATEMENT OF SUBJECT MATTER AND PURPOSE............................... 4 1.1. Procedure followed................................................................................................... 4 1.2. Purpose of the assessment report............................................................................ 4 2. OVERALL SUMMARY AND CONCLUSIONS............................................ 5 2.1. Presentation of the Active Substance.......................................................................5 2.1.1. Identity, Physico-Chemical Properties & Methods of Analysis..................................... 5 2.1.2. Intended Uses and Efficacy..................................................................................... 9 2.1.3. Classification and Labelling.................................................................................... 10 2.2. Summary of the Risk Assessment............................................................................10 2.2.1. Human Health Risk Assessment............................................................................. 10 2.2.1.1. Hazard identification and effects assessment.................................................... 10 2.2.1.2. -
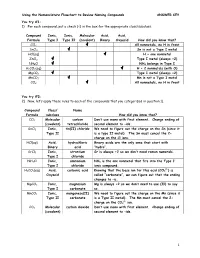
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Safe Handling and Disposal of Chemicals Used in the Illicit Manufacture of Drugs
Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel.: (+43-1) 26060-0, Fax: (+43-1) 26060-5866, www.unodc.org Guidelines for the Safe handling and disposal of chemicals used in the illicit manufacture of drugs United Nations publication USD 26 Printed in Austria ISBN 978-92-1-148266-9 Sales No. E.11.XI.14 ST/NAR/36/Rev.1 V.11-83777—September*1183777* 2011—300 Guidelines for the Safe handling and disposal of chemicals used in the illlicit manufacture of drugs UNITED NATIONS New York, 2011 Symbols of United Nations documents are composed of letters combined with figures. Mention of such symbols indicates a reference to a United Nations document. ST/NAR/36/Rev.1 UNITED NATIONS PUBLICATION Sales No. E.11.XI.14 ISBN 978-92-1-148266-9 eISBN 978-92-1-055160-1 © United Nations, September 2011. All rights reserved. The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. Requests for permission to reproduce this work are welcomed and should be sent to the Secretary of the Publications Board, United Nations Headquarters, New York, N.Y. 10017, U.S.A. or also see the website of the Board: https://unp.un.org/Rights.aspx. Governments and their institutions may reproduce this work without prior authoriza- tion but are requested to mention the source and inform the United Nations of such reproduction. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes.