NAMING ACIDS Acids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Naming Polyatomic Ions and Acids Oxyanions
Oxyanions Naming Polyatomic Ions and Oxyanions Acids Oxyanions- negative ions containing Oxyanions may contain the prefix oxygen. “hypo-”, less than, or “per-”, more than. These have the suffix “-ate” or “-ite” For example - “-ate” means it has more oxygen atoms ClO4 Perchlorate bonded, “-ite” has less - ClO3 Chlorate For example - ClO2 Chlorite 2- SO4 sulfate ClO- Hypochlorite 2- SO3 sulfite Acids Naming acids Naming Acids Certain compounds produce H+ ions in Does it contain oxygen? water, these are called acids. If it does not, it gets the prefix “hydro-” and If it does contain an oxyanion, then the suffix “-ic acid” replace the ending. You can recognize them because the neutral compound starts with “H”. HCl If the ending was “–ate”, add “-ic acid” Hydrochloric acid If the ending was “–ite”, add “-ous acid” For example HCl, H2SO4, and HNO3. HF Don’t confuse a polyatomic ion with a H2SO4 Sulfuric Acid Hydrofluoric acid neutral compound. H2SO3 Sulfurous Acid HCN HCO - is hydrogen carbonate, not an acid. 3 Hydrocyanic acid Examples Examples Nomenclature (naming) of Covalent compounds HNO3 HNO3 Nitric Acid HI HI Hydroiodic acid H3AsO4 H3AsO4 Arsenic Acid HClO2 HClO2 Chlorous Acid 1 Determining the type of bond Covalent bonding is very Covalent bonding is very First, determine if you have an ionic different from ionic naming compound or a covalent compound. similar to ionic naming A metal and a nonmetal will form an You always name the one that is least Ionic names ignored the subscript ionic bond. electronegative first (furthest from because there was only one possible Compounds with Polyatomic ions form fluorine) ratio of elements. -

Chemistry: the Molecular Nature of Matter and Change
REVISED CONFIRMING PAGES The Components of Matter 2.1 Elements, Compounds, and 2.5 The Atomic Theory Today 2.8 Formula, Name, and Mass of Mixtures: An Atomic Overview Structure of the Atom a Compound 2.2 The Observations That Led to Atomic Number, Mass Number, and Binary Ionic Compounds an Atomic View of Matter Atomic Symbol Compounds That Contain Polyatomic Mass Conservation Isotopes Ions Definite Composition Atomic Masses of the Elements Acid Names from Anion Names Multiple Proportions 2.6 Elements: A First Look at the Binary Covalent Compounds The Simplest Organic Compounds: Dalton’s Atomic Theory Periodic Table 2.3 Straight-Chain Alkanes Postulates of the Atomic Theory Organization of the Periodic Table Masses from a Chemical Formula How the Atomic Theory Explains Classifying the Elements Representing Molecules with a the Mass Laws Compounds: An Introduction 2.7 Formula and a Model to Bonding 2.4 The Observations That Led to Mixtures: Classification and the Nuclear Atom Model The Formation of Ionic Compounds 2.9 Separation Discovery of the Electron and The Formation of Covalent An Overview of the Components of Its Properties Compounds Matter Discovery of the Atomic Nucleus (a) (b) (right) ©Rudy Umans/Shutterstock IN THIS CHAPTER . We examine the properties and composition of matter on the macroscopic and atomic scales. By the end of this chapter, you should be able to • Relate the three types of matter—elements or elementary substances, compounds, and mixtures—to the simple chemical entities that they comprise—atoms, ions, and molecules; -

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Acetate C2H3O2 Or CH3COO Or CH3CO2 Ammonium NH4
Name:_____________________ Memorization For AP Chem Polyatmic Ions AP Chemistry Polyatomic Ion list - - - Acetate C2H3O2 or CH 3COO or CH 3CO 2 + Ammonium NH 4 - Ammonium Carbonate NH 4CO 3 Cyanide CN - 2- Carbonate CO 3 2- Dichromate Cr 2O7 - Dihydrogen phosphate H2PO 4 - Hydrogen carbonate HCO 3 - Hydrogen sulfate HSO 4 - Hydronium H3O Hydroxide OH - 2- Monohydrogen Phosphate HPO 4 - Nitrate NO 3 - Nitrite NO 2 2- Oxalate C2O4 - Permanganate MnO 4 3- Phosphate PO 4 2- Sulfate SO 4 2- Sulfite SO 3 Hypochlorite ClO - - Chlorite ClO 2 - Chlorate ClO 3 - Perchlorate ClO 4 2- Chromate CrO 4 2- Silicate SiO 3 Thiocyanate SCN - 2- Thiosulfate S2O3 - Bromate BrO 3 2- Selenate SeO 4 - Bisulfite HSO 3 Name:_____________________ Memorization For AP Chem Rules for Naming an Acid When the name of the anion ends in –ide, the acid name begins with the prefix hydro-, the stem of the anion has the suffix –ic and it is followed by the word acid. -ide becomes hydro _____ic Acid Cl- is the Chloride ion so HCl = hydrochloric acid When the anion name ends in –ite, the acid name is the stem of the anion with the suffix –ous, followed by the word acid. -ite becomes ______ous Acid - ClO 2 is the Chlorite ion so HClO 2 = Chlorous acid. When the anion name ends in –ate, the acid name is the stem of the anion with the suffix –ic, followed by the word acid. -ate becomes ______ic Acid - ClO 3 is the Chlorate ion so HClO 3 = Chloric acid. Rules for Naming Ionic Compounds 1. -
Proceedings of the Indiana Academy of Science
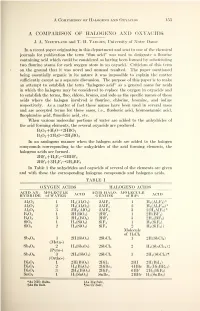
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

Monoatomic Ions You Should Know
Monoatomic ions you should know Monoatomic Cations Elements that form a single cation Elements that form two or more cations Ion Name Ion Name Common Name + 2+ H hydrogen ion /proton Cr chromium(II) ion Li+ lithium ion Cr3+ chromium(III) ion + 2+ Na sodium ion Fe iron(II) ion ferrous ion + 3+ K potassium ion Fe iron(III) ion ferric ion + 2+ Rb rubidium ion Ni nickel(II) + 3+ Cs cesium ion Ni nickel(III) 2+ + Be beryllium ion Cu copper(I) ion cuprous ion 2+ 2+ Mg magnesium ion Cu copper(II) ion cupric ion 2+ 2+ Ca calcium ion Hg2 mercury(I) ion* mercurous ion Sr2+ strontium ion Hg2+ mercury(II) ion mercuric ion Ba2+ barium ion Sn2+ tin(II) ion stannous ion Al3+ aluminum ion Sn4+ tin(IV) ion stannic ion Zn2+ zinc ion Pb2+ lead(II) ion plumbous ion Ag+ silver ion Pb4+ lead(IV) ion plumbic ion Cd2+ cadmium ion *this is actually a polyatomic ion Monoatomic Anions (all form only single ion) Ion Name Ion Name N3- nitride ion H- hydride ion P3- phosphide ion F- fluoride ion O2- oxide ion Cl- chloride ion S2- sulfide ion Br- bromide ion I- iodide ion Polyatomic ions you should know Formula Name Formula Name - 2- C2H3O2 acetate ion CO3 carbonate ion + - NH4 ammonium ion HCO3 bicarbonate ion 2- 2- CrO4 chromate ion SO4 sulfate ion Cr O 2- dichromate ion HSO - bisulfate ion 2 7 4 - 2- CN cyanide ion SO3 sulfite ion - - HO hydroxide ion HSO3 bisulfite ion 2- - C2O4 oxalate ion ClO4 perchlorate ion - - MnO4 permanganate ion ClO3 chlorate ion - - NO3 nitrate ion ClO2 chlorite ion - - NO2 nitrite ion ClO hypochlorite ion PO 3- phosphate ion 4 2- HPO4 hydrogen phosphate ion - H2PO4 dihydrogen phosphate ion Acids you should know Hydrohalic acids Oxygen containing acids Formula Name Formula Name HF hydrofluoric acid H3PO4 phosphoric acid HCl hydrochloric acid H3PO3 phosphorous acid HBr hydrobromic acid H2SO4 sulfuric acid HI hydroiodic acid H2SO3 sulfurous acid H2CO3 carbonic acid HMnO4 permanganic acid HNO3 nitric acid HNO2 nitrous acid HC2H3O2 acetic acid H2C2O4 oxalic acid HClO4 perchloric acid HClO3 chloric acid HClO2 chlorous acid HClO hypochlorous acid . -

Significant Figures
For students and parents/guardians Table of Contents In the Elements Handbook, you’ll find use- ful information about the properties of the Elements Handbook . 901 main group elements from the periodic table. Hydrogen. 904 You’ll also learn about real-world applications Group 1: Alkali Metals. 906 for many of the elements. Group 2: Alkaline Earth Metals . 910 The Math Handbook helps you review and Groups 3–12: Transition Elements . 916 sharpen your math skills so you get the most Group 13: Boron Group . 922 out of understanding how to solve math prob- Group 14: Carbon Group . .926 lems involving chemistry. Reviewing the rules Group 15: Nitrogen Group . 932 Group 16: Oxygen Group . .936 for mathematical operations such as scientific Group 17: Halogen Group . 940 notation, fractions, and logarithms can also Group 18: Noble Gases . .944 help you boost your test scores. The reference tables are another tool that Math Handbook . .946 will assist you. The practice problems and Scientific Notation . .946 solutions are resources that will help increase Operations with Scientific Notation . 948 your comprehension. Square and Cube Roots . 949 Significant Figures . .949 Solving Algebraic Equations. 954 Dimensional Analysis . 956 Unit Conversion . 957 Drawing Line Graphs. 959 Using Line Graphs . .961 Ratios, Fractions, and Percents. 964 Operations Involving Fractions . .965 Logarithms and Antilogarithms. 966 Reference Tables. 968 R-1 Color Key. 968 R-2 Symbols and Abbreviations. 968 R-3 Solubility Product Constants . 969 R-4 Physical Constants . .969 R-5 Names and Charges of Polyatomic Ions . 970 R-6 Ionization Constants . .970 R-7 Properties of Elements. -
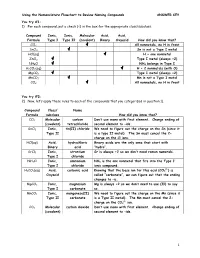
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes.