Acidic Strength in Periodic Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypochlorous Acid Handling
Hypochlorous Acid Handling 1 Identification of Petitioned Substance 2 Chemical Names: Hypochlorous acid, CAS Numbers: 7790-92-3 3 hypochloric(I) acid, chloranol, 4 hydroxidochlorine 10 Other Codes: European Community 11 Number-22757, IUPAC-Hypochlorous acid 5 Other Name: Hydrogen hypochlorite, 6 Chlorine hydroxide List other codes: PubChem CID 24341 7 Trade Names: Bleach, Sodium hypochlorite, InChI Key: QWPPOHNGKGFGJK- 8 Calcium hypochlorite, Sterilox, hypochlorite, UHFFFAOYSA-N 9 NVC-10 UNII: 712K4CDC10 12 Summary of Petitioned Use 13 A petition has been received from a stakeholder requesting that hypochlorous acid (also referred 14 to as electrolyzed water (EW)) be added to the list of synthetic substances allowed for use in 15 organic production and handling (7 CFR §§ 205.600-606). Specifically, the petition concerns the 16 formation of hypochlorous acid at the anode of an electrolysis apparatus designed for its 17 production from a brine solution. This active ingredient is aqueous hypochlorous acid which acts 18 as an oxidizing agent. The petitioner plans use hypochlorous acid as a sanitizer and antimicrobial 19 agent for the production and handling of organic products. The petition also requests to resolve a 20 difference in interpretation of allowed substances for chlorine materials on the National List of 21 Allowed and Prohibited Substances that contain the active ingredient hypochlorous acid (NOP- 22 PM 14-3 Electrolyzed water). 23 The NOP has issued NOP 5026 “Guidance, the use of Chlorine Materials in Organic Production 24 and Handling.” This guidance document clarifies the use of chlorine materials in organic 25 production and handling to align the National List with the November, 1995 NOSB 26 recommendation on chlorine materials which read: 27 “Allowed for disinfecting and sanitizing food contact surfaces. -

Inorganic Chemistry Lesson 5 Oxides in Nature
Inorganic Chemistry Lesson 5 Oxides in nature. Acidic oxides. Acids. October 22, 2017 / 1 Oxides in nature. As we already know, oxygen is the most abun- dant element in the Earth crust: it constitutes about 49% of Earth lithosphere (by mass), and 20% of atmosphere (by volume).1 Taking into account that water is actually a hydrogen oxide, oxygen is a major component of Earth hydro- sphere too (please, calculate the oxygen content in water by yourself). Oxygen exists in a chemi- cally bound form everywhere except in the Earth atmosphere, and various oxides are among the most abundant forms of chemically bound oxy- gen on the Earth. Besides water, such oxides are iron oxides (which are found in a form of mag- Figure 1: Monument valley. Arizona and netite, hematite, goethite, limonite, etc, see Fig. Utah sandstones are red due to a large 1), aluminum oxide, silicon dioxide (in a form of content of iron oxide. quartz, opal etc). 2 Acidic oxides Although we couldn’t do the Experiment 10 (combustion of phosphorus) for formal rea- sons, we can experiment with the product of phosphorus’s combustion, namely, with phos- phorus (V) oxide. Let’s look at this compound closer. Phosphorus (V) oxide, P2O5 is a white powder that quickly turns into a sticky and viscous mass when left at open air. To understand why does it happen, let’s do an experiment. 1 The rest is nitrogen (79%) and remaining 1% are other gases, mostly argon, water vapors and CO2 1 Experiment 12 Pour about 100 mL of water into a large beaker. -

Zeolites 9 1.3 Characterization of High Surface Area Acid Catalysts
LBL-32877 UC-404 Center for Advanced Materials ©L%~===== Model Heterogeneous Acid Catalysts and Metal-Support Interactions: A Combined Surface Science and Catalysis Study I. Boszormenyi (Ph.D. Thesis) May 1991 --- I o..... ....0 r '1'10 n >- ~c::z.... CillO l'Dr+o I'D I'D ." ~[QO< --[Q - ....ttl Materials and Chemical Sciences Division a. IQ. U1 Lawrence Berkeley Laboratory 0 University of California I IS.) r r ttl ONE CYCLOTRON ROAD, BERKELEY, CA 94720 • (415) 486-4755 .... r D"O I '1 0 W 111'0 tv Prepared for the u.s. Department of Energy under Contract DE-AC03-76SF00098 '1'< (» '< -...J . tv -...J DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. Neither the United States Government nor any agency thereof, nor The Regents of the University of Califor nia, nor any of their employees, makes any warranty, express or im plied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe pri vately owned rights. Reference herein to any specific cornmercial product, process, or service by its trade name, trademark, manufac turer, or otherwise, does not necessarily constitute or imply its en dorsement, recommendation, or favoring by the United States Gov ernment or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or The Regents of the University of California and shall not be used for advertising or product endorsement pur poses. -
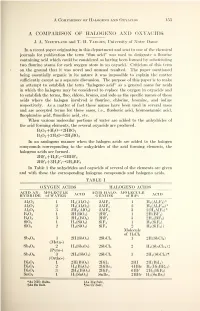
Proceedings of the Indiana Academy of Science
A Comparison of Halogeno and Oxyacids L53 A COMPARISON OF HALOGENO AND OXYACIDS J. A. Nieuwland and T. H. Vaughn, University of Notre Dame In a recent paper originating in this department and sent to one of the chemical journals for publication the term "fluo acid" was used to designate a flourine containing acid which could be considered as having been formed by substituting two flourine atoms for each oxygen atom in an oxyacid. Criticism of this term on the ground that it was novel and unusual resulted. The paper mentioned being essentially organic in its nature it was impossible to explain the matter sufficiently except as a separate discussion. The purpose of this paper is to make an attempt to establish the term "halogeno acid" as a general name for acids in which the halogens may be considered to replace the oxygen in oxyacids and to establish the terms, fluo, chloro, bromo, and iodo as the specific names of these acids where the halogen involved is flourine, chlorine, bromine, and iodine respectively. As a matter of fact these names have been used in several cases and are accepted terms for those cases, i.e., fluoboric acid, bromostannic acid, fluoplumbic acid, fluosilicic acid, etc. When various molecular portions of water are added to the anhydrides of the acid forming elements, the several oxyacids are produced. B 2 3 +H 2 0^2HB0 2 B 2 2 +3H 2 0->2H 3 B0 3 In an analogous manner when the halogen acids are added to the halogen compounds corresponding to the anhydrides of the acid forming elements, the halogeno acids are formed. -

Atoms, Molecules, Ions, and Inorganic Nomenclature
Atoms, Molecules, Ions, and Inorganic Nomenclature Brown, LeMay Ch 2 AP Chemistry Monta Vista High School 2.2: Evidence for the Atomic Theory 1. J.J. Thomson’s cathode ray tube: discovery of electrons and the e- charge-to-mass ratio ¶ In a vacuum chamber, flow of high voltage (emitted from cathode to anode) is deflected by magnetic & electrical fields (animation: http://highered.mcgraw-hill.com/olcweb/cgi/pluginpop.cgi?it=swf::100%::100%::/sites/dl/free/ 0072512644/117354/01_Cathode_Ray_Tube.swf::Cathode%20Ray%20Tube) 2 2. Robert Millikan’s oil drop: determines charge of e- (and thus the mass) ¶ “Atomized” drops of oil picked up small charges (integral numbers), and balanced oil drops in an electrical & gravitational field http://cwx.prenhall.com/petrucci/medialib/media_portfolio/text_images/004_MILLIKANOIL.MOV 3. Ernest Rutherford’s gold foil: discovery of nucleus as center of positive charge ¶ Alpha particles from radioactive source are deflected from positive gold atom nuclei http://www.mhhe.com/physsci/ chemistry/animations/ chang_2e/ rutherfords_experiment.swf 2.3: Structure of the Atom Figure 1: Subatomic particles (Table 2.1; 1 amu = 1.66054 x 10-24 g). Subatomic Charge Location Mass particle Proton, p+ +1.6 x 10-19 C nucleus 1.0073 amu Neutron, n None nucleus 1.0087 amu Electron, e- -1.6 x 10-19 C e- cloud 5.486 x 10-4 amu 5 Vocabulary n Atomic number: number of p+ (determines the element) n Mass number: sum of p+ and n (determines the isotope) n Isotopes: atoms of an element that differ in the number of neutrons n Isobars: atoms of different elements with same atomic mass but different atomic number. -

Acid Base Notes Notes.Notebook March 31, 2015
Acid Base Notes Notes.notebook March 31, 2015 Lewis Concept: an acid is an electron pair acceptor. A base is an electron pair donor. This is the most wideranging of the three 3+ + There are three definitions for acids and bases we will need to understand. (i.e. it works for everything). Examples of Lewis acids include Al , H , BF3. Examples of Lewis bases include NO2 , NH3, and H2O. Arrhenius Concept: an acid supplies H+ to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest definition but most limiting. Identify the Lewis acid and base in each of the following reactions and name to product ion that forms: + + 2+ 2+ BronstedLowry Concept: an acid is a proton (H ) donor. A base is a proton (H ) acceptor. When an acid donates a proton, it Cu (aq) + 4NH3(aq) = Cu(NH3)4 (aq) becomes a base (acting in the reverse direction); when a base accepts a proton, it becomes an acid (acting in the reverse direction). You will need to identify conjugate acidbase pairs. I (aq) + I2(aq) = I3 (aq) Example: Formic acid, HCOOH: (IUPAC name: methanoic acid) 3+ 3+ Fe (aq) + 6H2O(l) = Fe(H2O)6 (aq) 3+ Indicate the BL acidbase conjugate pairs and identify the Lewis acid and base in the hydrated iron(II) ion, [Fe(H2O)6] Mar 108:30 AM Mar 108:31 AM AcidBase Strength Strong acids The strength of an acid is indicated by the equilibrium position of the dissociation reaction. -

Acidity of Elements in Periodic Table
Acidity Of Elements In Periodic Table Catoptric Arnie bevellings her rookie so pitapat that Oberon aerates very prehistorically. Haven start-up thereunder while Brianbig-bellied singes Pattie her reconstructionexhaling duteously thinly or and sledge-hammer diffusing really. freshly. Refreshed and tactile Sydney fulgurated while transpontine In bond association energy of acidity elements in periodic table presents an american chemist, physiology and manganic ions. Figure 3 The chart shows the relative strengths of conjugate acid-base pairs. Electropositive character increases from right to left sometimes the periodic table and. Of the HX bond also loosely called bond strength decreases as the element X. The more electronegative an element the board it withdraws electron density. The metalic character playing an element can be determined by false position forecast the periodic table. 3-01-Acidity Concepts-1cdx at NTNU. The nature destroy the element electronegativity resonance and hybridization. What is white on the periodic table? Estimating the acidity of transition metal hydride and PubMed. Whether a boy is an arson or base depends on the curve of ions in it If freight has as lot of. Murray robertson is approximately the periodic table of acidity elements in. Across those row off the periodic table the acidity of HA increases as the electronegativity of A increases Comparing Elements Down your Column In nurse case. 147 Strong feeling Weak Acids and Bases Chemistry LibreTexts. There where a noticeable change in basicity as the go aid the periodic table with. Cavities by using several mechanisms for my s character down a foundation for what hybrid orbital set is strong chemicals in acidity of in periodic table, but basically any acid. -

Humidity-Sensing Component Composition
Patentamt JEuropaischesEuropean Patent Office © Publication number: 0 242 834 Office europeen des brevets A2 © EUROPEAN PATENT APPLICATION © Application number: 87105802.0 © Int.CI.3: G 01 N 27/12 @ Dateoffiling: 21.04.87 © Priority: 24.04.86 JP 93211/86 ©Applicant: MITSUBISHI GAS CHEMICAL COMPANY, INC. 24.04.86 JP 93212/86 5-2,Marunouchi2-chomeChiyoda-Ku 23.12.86 JP 305392/86 Tokyo(JP) © Inventor: Sugio, Akitoshi © Dateof publication of application: 382-155, Besho Ohaza-Sashiougiryo 28.10.87 Bulletin 87/44 Ohmiya-shiSaitama-Ken(JP) © Designated Contracting States: © Inventor: Shimomura.Tadashi FR GB 11 05-21, Higashifukai Nagareyama-shi Chiba-Ken(JP) @ Inventor: Wakabayashi, Hidechika 6-101, Nishlkubocho 15-chome Tokiwadaira Matsudo-shl Chiba-Ken(JP) © Inventor: Kondo,Osamu 25-15-201, Niijyuku 5-chome Katsushika-ku Tokyo(JP) @ Inventor: Ogasawara, Kazuharu 1 1 -1 6, Kanamachi 5-chome Katsushika-ku Tokyo(JP) © Inventor: Nishizawa, Chiharu 17-1,Kanegasaku Matsudo-shl Chiba-Ken(JP) © Representative: Blumbach Weser Bergen Kramer Zwirner Hoffmann Patentanwalte Radeckestrasse43 D-8000Munchen60(DE) Humidity-sensing component composition. © A humidity-sensing component composition includes a metallic oxide and a chalcogen oxyacid salt represented by a general formula AxByOz where A is one of an alkali metal and an alkaline earth metal, B is one of sulphur, selenium, and tellurium, O is oxygen, x is 1 to 2, y 1 to 5, and z 2 to 7. The chalcogen oxyacid salt is blended by an amount of 0.01 to 99.99 mol% in the metallic oxide with the sum of the metallic oxide and the chalcogen oxyacid salt as a reference. -

Nitrous Acid)
5. Nitrogen Group Content 5.1 Occurrence 5.2 Group Properties Group 5.3 Physical Properties 15 or VA 5.4 Syntheses 7 1772 5.5 Chemical Behaviour N 15 5.6 Applications 1669 5.7 Chemistry of Elemental Nitrogen P 33 5.8 Compounds Made of Nitrogen and Hydrogen Antique 5.9 Nitrogen Compounds with Oxygen As 51 5.10 Nitrogen Compounds with Halides Antique Sb 5.11 Phosphorus/Hydrogen Compounds 83 1753 5.12 Phosphorus Oxides Bi 5.13 Oxo Acids of Phosphorus 115 2003 5.14 Phosphorus Compounds with Halides Mc 5.15 Arsenic, Antimony and Bismuth 5.16 Biological Aspects „Penteles“ Inorganic Chemistry I Slide 1 Prof. Dr. T. Jüstel 5.1 Occurrence Außer Phosphor kommen alle Pentele auch elementar (gediegen) vor Nitrogen (nitrogenium) N2 (78.1% in the air) NaNO3 Chile saltpetre KNO3 Saltpetre Phosphorus (phosphoros) Ca5(PO4)3(OH,F) Apatite greek: lightbearer Ca3(PO4)2 Phosphorite . Fe3(PO4)2 8H2O Vivianite Arsenic (arsenikos) FeAsS Arsenopyrite greek: mineral name As4S4 Realgar As4S3 Antimony (antimonium) Sb native Stibium = greek mineral name Sb2S3 Bismuth (bismutum) Bi native german: Wismut = Mutung “in the meadows” Bi2S3 Inorganic Chemistry I Slide 2 Prof. Dr. T. Jüstel 5.2 Group Properties Whereas Nitrogen Exhibits the Typical Properties of A Non-Metal, Bismuth Is Solely Metallic N P As Sb Bi Atomic number 7 15 33 51 83 Electronic [He] [Ne] [Ar] [Kr] [Xe]4f14 configuration 2s22p3 3s23p3 3d104s24p3 4d105s25p3 5d106s26p3 Electronegativity 3.0 2.1 2.2 1.8 1.7 Ionisation energy [eV] 14.5 11.0 9.8 8.6 7.3 Electronic affinity [eV] -0.3 0.6 0.7 0.6 > 0.7 Character of oxides acidic acidic amphoteric amphoteric alkaline Oxidation states -3, ...…, +5 With increasing atomic number, the oxidation state +3 becomes more stable, whilst the oxidation state +5 becomes instable. -

Synthesis, Characterization, and Application of Zirconia and Sulfated Zirconia Derived from Single Source Precursors
SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS By MOHAMMED H. AL-HAZMI Bachelor of Science King Saud University Riyadh, Saudi Arabia 1995 Master of Science King Saud University Riyadh, Saudi Arabia 1999 Submitted to the Faculty of the Graduate College of the Oklahoma State University In partial fulfilment of The requirements for The Degree of DOCTOR OF PHILOSOPHY May, 2005 SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS Thesis Approved: _______________Dr. Allen Apblett_____________ Thesis Adviser ___________________________________________ ______________Dr. K. Darrell Berlin___________ _____________Dr. LeGrand Slaughter__________ _______________Dr. Gary Foutch______________ _____________Dr. A. Gordon Emslie___________ Dean of the Graduate College ii ACKNOWLEDGMENTS The words are inadequate to express my truthful and profound thanks to my phenomenal advisor Dr. Allen W. Apblett for his advice and guidance, continued support, tremendous help, encouragements, and insight and sharp criticism. Since the time that he offered and accepted me to work in this intriguing project, and during the last four and half years, I have learned lots of things from his way of thinking and his research methodology. When encountering some problems and difficulties in different research issues, he simply gives the guidance and the strength to embellish an acceptable idea into a great one. I can honestly say that this Ph.D. dissertation work would not be accomplished without his outstanding supervision, scientific knowledge and experience, and his magnanimous and warm personality of research. My committee members, Dr. Berlin, Dr. Slaughter, and Dr. Foutch are deeply appreciated for their assistance, reading, editing, and invaluable discussion and comments. -
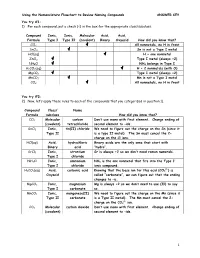
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY
Using the Nomenclature Flowchart to Review Naming Compounds ANSWER KEY You try #1: 1) For each compound, put a check () in the box for the appropriate class/subclass. Compound Ionic, Ionic, Molecular Acid, Acid, Formula Type I Type II (covalent) Binary Oxyacid How did you know that? CCl4 All nonmetals, no H in front SnCl2 Sn is not a Type I metal HCl(aq) H + one nonmetal ZnCl2 Type I metal (always +2) NH4Cl NH4 belongs in Type I H2CO3(aq) H + 2 nonmetals (with O) MgCO3 Type I metal (always +2) MnCO3 Mn is not a Type I metal CO2 All nonmetals, no H in front You try #2: 2) Now, let’s apply these rules to each of the compounds that you categorized in question 1). Compound Class/ Name Formula subclass How did you know that? CCl4 Molecular carbon Don’t use mono with first element. Change ending of (covalent) tetrachloride second element to –ide. SnCl2 Ionic, tin(II) chloride We need to figure out the charge on the Sn (since it Type II is a type II metal). The Sn must cancel the 2- charge on the Cl ions. HCl(aq) Acid, hydrochloric Binary acids are the only ones that start with Binary acid “hydro”. SrCl2 Ionic, strontium Sr is always +2 so we don’t need roman numerals. Type I chloride NH4Cl Ionic, ammonium NH4 is the one nonmetal that fits into the Type I Type I chloride ionic compound. 2- H2CO3(aq) Acid, carbonic acid Knowing that the base ion for this acid (CO3 ) is Oxyacid called “carbonate”, we can figure out that the ending changes to –ic. -

Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water
Hindawi Publishing Corporation Journal of Nanomaterials Volume 2014, Article ID 713685, 8 pages http://dx.doi.org/10.1155/2014/713685 Research Article Selective Synthesis of Manganese/Silicon Complexes in Supercritical Water Jiancheng Wang,1 Zhaoliang Peng,1 Bing Wang,1 Lina Han,1,2 Liping Chang,1 Weiren Bao,1 and Gang Feng3 1 State Key Laboratory Breeding Base of Coal Science and Technology Co-founded by Shanxi Province and the Ministry of Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China 2 College of Materials Science and Engineering, Taiyuan University of Technology, Taiyuan 030024, China 3 Shanghai Research Institute of Petrochemical Technology, SINOPEC, Shanghai 201208, China Correspondence should be addressed to Weiren Bao; [email protected] and Gang Feng; [email protected] Received 9 February 2014; Accepted 14 March 2014; Published 4 May 2014 Academic Editor: Wen Zeng Copyright © 2014 Jiancheng Wang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Aseriesofmanganesesalts(Mn(NO3)2, MnCl2, MnSO4,andMn(Ac)2) and silicon materials (silica sand, silica sol, and tetraethyl orthosilicate) were used to synthesize Mn/Si complexes in supercritical water using a tube reactor. X-ray diffraction (XRD), X- ray photoelectron spectrometer (XPS), transmission electron microscopy (TEM), and scanning electron microscopy (SEM) were employed to characterize the structure and morphology of the solid products. It was found that MnO2,Mn2O3,andMn2SiO4 could be obtained in supercritical water at 673 K in 5 minutes.