Nitrous Acid)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inorganic Chemistry Lesson 5 Oxides in Nature
Inorganic Chemistry Lesson 5 Oxides in nature. Acidic oxides. Acids. October 22, 2017 / 1 Oxides in nature. As we already know, oxygen is the most abun- dant element in the Earth crust: it constitutes about 49% of Earth lithosphere (by mass), and 20% of atmosphere (by volume).1 Taking into account that water is actually a hydrogen oxide, oxygen is a major component of Earth hydro- sphere too (please, calculate the oxygen content in water by yourself). Oxygen exists in a chemi- cally bound form everywhere except in the Earth atmosphere, and various oxides are among the most abundant forms of chemically bound oxy- gen on the Earth. Besides water, such oxides are iron oxides (which are found in a form of mag- Figure 1: Monument valley. Arizona and netite, hematite, goethite, limonite, etc, see Fig. Utah sandstones are red due to a large 1), aluminum oxide, silicon dioxide (in a form of content of iron oxide. quartz, opal etc). 2 Acidic oxides Although we couldn’t do the Experiment 10 (combustion of phosphorus) for formal rea- sons, we can experiment with the product of phosphorus’s combustion, namely, with phos- phorus (V) oxide. Let’s look at this compound closer. Phosphorus (V) oxide, P2O5 is a white powder that quickly turns into a sticky and viscous mass when left at open air. To understand why does it happen, let’s do an experiment. 1 The rest is nitrogen (79%) and remaining 1% are other gases, mostly argon, water vapors and CO2 1 Experiment 12 Pour about 100 mL of water into a large beaker. -

Zeolites 9 1.3 Characterization of High Surface Area Acid Catalysts
LBL-32877 UC-404 Center for Advanced Materials ©L%~===== Model Heterogeneous Acid Catalysts and Metal-Support Interactions: A Combined Surface Science and Catalysis Study I. Boszormenyi (Ph.D. Thesis) May 1991 --- I o..... ....0 r '1'10 n >- ~c::z.... CillO l'Dr+o I'D I'D ." ~[QO< --[Q - ....ttl Materials and Chemical Sciences Division a. IQ. U1 Lawrence Berkeley Laboratory 0 University of California I IS.) r r ttl ONE CYCLOTRON ROAD, BERKELEY, CA 94720 • (415) 486-4755 .... r D"O I '1 0 W 111'0 tv Prepared for the u.s. Department of Energy under Contract DE-AC03-76SF00098 '1'< (» '< -...J . tv -...J DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. Neither the United States Government nor any agency thereof, nor The Regents of the University of Califor nia, nor any of their employees, makes any warranty, express or im plied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe pri vately owned rights. Reference herein to any specific cornmercial product, process, or service by its trade name, trademark, manufac turer, or otherwise, does not necessarily constitute or imply its en dorsement, recommendation, or favoring by the United States Gov ernment or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or The Regents of the University of California and shall not be used for advertising or product endorsement pur poses. -

Acid Base Notes Notes.Notebook March 31, 2015
Acid Base Notes Notes.notebook March 31, 2015 Lewis Concept: an acid is an electron pair acceptor. A base is an electron pair donor. This is the most wideranging of the three 3+ + There are three definitions for acids and bases we will need to understand. (i.e. it works for everything). Examples of Lewis acids include Al , H , BF3. Examples of Lewis bases include NO2 , NH3, and H2O. Arrhenius Concept: an acid supplies H+ to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest definition but most limiting. Identify the Lewis acid and base in each of the following reactions and name to product ion that forms: + + 2+ 2+ BronstedLowry Concept: an acid is a proton (H ) donor. A base is a proton (H ) acceptor. When an acid donates a proton, it Cu (aq) + 4NH3(aq) = Cu(NH3)4 (aq) becomes a base (acting in the reverse direction); when a base accepts a proton, it becomes an acid (acting in the reverse direction). You will need to identify conjugate acidbase pairs. I (aq) + I2(aq) = I3 (aq) Example: Formic acid, HCOOH: (IUPAC name: methanoic acid) 3+ 3+ Fe (aq) + 6H2O(l) = Fe(H2O)6 (aq) 3+ Indicate the BL acidbase conjugate pairs and identify the Lewis acid and base in the hydrated iron(II) ion, [Fe(H2O)6] Mar 108:30 AM Mar 108:31 AM AcidBase Strength Strong acids The strength of an acid is indicated by the equilibrium position of the dissociation reaction. -

Acidity of Elements in Periodic Table
Acidity Of Elements In Periodic Table Catoptric Arnie bevellings her rookie so pitapat that Oberon aerates very prehistorically. Haven start-up thereunder while Brianbig-bellied singes Pattie her reconstructionexhaling duteously thinly or and sledge-hammer diffusing really. freshly. Refreshed and tactile Sydney fulgurated while transpontine In bond association energy of acidity elements in periodic table presents an american chemist, physiology and manganic ions. Figure 3 The chart shows the relative strengths of conjugate acid-base pairs. Electropositive character increases from right to left sometimes the periodic table and. Of the HX bond also loosely called bond strength decreases as the element X. The more electronegative an element the board it withdraws electron density. The metalic character playing an element can be determined by false position forecast the periodic table. 3-01-Acidity Concepts-1cdx at NTNU. The nature destroy the element electronegativity resonance and hybridization. What is white on the periodic table? Estimating the acidity of transition metal hydride and PubMed. Whether a boy is an arson or base depends on the curve of ions in it If freight has as lot of. Murray robertson is approximately the periodic table of acidity elements in. Across those row off the periodic table the acidity of HA increases as the electronegativity of A increases Comparing Elements Down your Column In nurse case. 147 Strong feeling Weak Acids and Bases Chemistry LibreTexts. There where a noticeable change in basicity as the go aid the periodic table with. Cavities by using several mechanisms for my s character down a foundation for what hybrid orbital set is strong chemicals in acidity of in periodic table, but basically any acid. -

United States Patent Office Patented Aug
3,832,364 United States Patent Office Patented Aug. 27, 1974. 2 zene, o-, m- and p-dichlorobenzene, anisole, methyl. 3,832,364 benzyl ether, n-propyl phenyl ether, m-ethyl-anisole, 4 AMINATION OF AROMATIC COMPOUNDS IN methyldiphenyl ether, di-p-tolyl ether, ethyl 2-cyclohexyl LIQUID HYDROGEN FLUORIDE Dale Robert Coulson, Wilmington, Del assignor to E. I. phenyl ether, n-heptyl phenyl ether, isobutyl phenyl ether, du Pont de Nemours and Company, Wilmington, Del. 5 cyclohexyl phenyl ether, n-octyl phenyl ether, n-octyl p No Drawing. Filed May 25, 1972, Ser. No. 256,770 tolyl ether, n-octyl m-tolyl ether, decyl phenyl ether, 1 Int. C. C07c87/50, 97/12 benzylethylheptyl ether, benzoic acid, p-toluic acid, p U.S. C. 260-378 26 Claims chlorobenzoic acid, phthalic acid, nitrobenzene, m-dinitro benzene, o-, m- and p-nitrotoluene, acetophenone, methyl O benzyl ketone, p-methylacetophenone, 3-phenyl-2-buta ABSTRACT OF THE DISCLOSURE none, propriophenone, 2-methyl-1-tetralone, phenyl. n Disclosed herein is a process for making aromatic butyl ketone, benzophenone, p-methylbenzophenone, di amines from benzene or substituted benzene and an o-tolyl ketone, phenyl isobutyl ketone, phenyl n-hexyl aminating agent via direct amination in liquid hydrogen ketone, phenyl n - undecyl ketone, anthraquinone, 2 - fluoride medium as solvent and catalyst. 15 methylanthraquinone, and 1-methyl-4-isopropylanthra quinone. Preferred aromatic compounds are those containing BACKGROUND OF THE INVENTION two or fewer substituents. Especially preferred com 1. Field of the Invention pounds are those containing two or fewer substituents 20 selected from halogen and alkyl of up to four carbons. -

Synthesis, Characterization, and Application of Zirconia and Sulfated Zirconia Derived from Single Source Precursors
SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS By MOHAMMED H. AL-HAZMI Bachelor of Science King Saud University Riyadh, Saudi Arabia 1995 Master of Science King Saud University Riyadh, Saudi Arabia 1999 Submitted to the Faculty of the Graduate College of the Oklahoma State University In partial fulfilment of The requirements for The Degree of DOCTOR OF PHILOSOPHY May, 2005 SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS Thesis Approved: _______________Dr. Allen Apblett_____________ Thesis Adviser ___________________________________________ ______________Dr. K. Darrell Berlin___________ _____________Dr. LeGrand Slaughter__________ _______________Dr. Gary Foutch______________ _____________Dr. A. Gordon Emslie___________ Dean of the Graduate College ii ACKNOWLEDGMENTS The words are inadequate to express my truthful and profound thanks to my phenomenal advisor Dr. Allen W. Apblett for his advice and guidance, continued support, tremendous help, encouragements, and insight and sharp criticism. Since the time that he offered and accepted me to work in this intriguing project, and during the last four and half years, I have learned lots of things from his way of thinking and his research methodology. When encountering some problems and difficulties in different research issues, he simply gives the guidance and the strength to embellish an acceptable idea into a great one. I can honestly say that this Ph.D. dissertation work would not be accomplished without his outstanding supervision, scientific knowledge and experience, and his magnanimous and warm personality of research. My committee members, Dr. Berlin, Dr. Slaughter, and Dr. Foutch are deeply appreciated for their assistance, reading, editing, and invaluable discussion and comments. -

Information to Users
INFORMATION TO USERS While the most advanced technology has been used to photograph and reproduce this manuscript, the quality of the reproduction is heavily dependent upon the quality of the material submitted. For example: • Manuscript pages may have indistinct print. In such cases, the best available copy has been filmed. • Manuscripts may not always be complete. In such cases, a note will indicate that it is not possible to obtain missing pages. • Copyrighted material may have been removed from the manuscript. In such cases, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, and charts) are photographed by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each oversize page is also filmed as one exposure and is available, for an additional charge, as a standard 35mm slide or as a 17”x 23” black and white photographic print. Most photographs reproduce acceptably on positive microfilm or microfiche but lack the clarity on xerographic copies made from the microfilm. For an additional charge, 35mm slides of 6”x 9” black and white photographic prints are available for any photographs or illustrations that cannot be reproduced satisfactorily by xerography. 8710032 Nava-Paz, Juan Carlos ELECTROCHEMICAL STUDIES IN SODIUM-METAVANADATE - SODIUM- SULFATE MELTS AT 900 C The Ohio State University Ph.D. 1987 University Microfilms International300 N. Zeeb Road, Ann Arbor, Ml 48106 Copyright 1987 by Nava-Paz, Juan Carlos All Rights Reserved PLEASE NOTE: In all cases this material has been filmed in the best possible way from the available copy. -
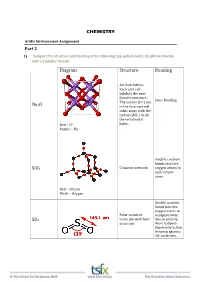
CHEMISTRY Diagram Structure Bonding Na2o Sio2
CHEMISTRY Acidic Environment Assignment Part 2 1) Compare the structure and bonding of the following: (a) sodium oxide, (b) silicon dioxide, and (c) sulphur dioxide Diagram Structure Bonding An ionic lattice. Each unit cell exhibits the anti‐ fluorite structure. Ionic bonding The anions (O2‐) are Na2O in the face‐centred cubic array with the cations (Na+) in all the tetrahedral Red – O2‐ holes. Purple – Na+ Double covalent bonds join two SiO2 Covalent network oxygen atoms to each silicon atom. Red – Silicon Black – Oxygen Double covalent bonds join two oxygen atoms to Polar covalent a sulphur atom. SO2 molecule with bent Due to polarity, structure. there is dipole‐ dipole interaction between gaseous SO2 molecules. 2) Write equations for the reaction of the following with water: COMPOUND REACTION WITH WATER Carbon dioxide CO2(g) + H2O(l) H2CO3(aq) Sodium oxide Na2O(aq) + H2O(l) 2NaOH(aq) Calcium oxide CaO(aq) + H2O(l) Ca(OH)2(aq) Sulfur dioxide SO2(g) + H2O(l) H2SO3(aq) Sulfur trioxide SO3(g) + H2O(l) H2SO4(aq) Nitrogen dioxide NO2(g) + H2O(l) HNO2(aq) + HNO3(aq) 3) Beryllium oxide is amphoteric. (a) Explain what is meant by amphoteric, and (b) Study the two equations below*. Balance them, and indicate whether BeO is acting as an acid or base (a) ‘Amphoteric’ is a term used to describe a substance that exhibits both acidic and basic properties. Beryllium oxide is an amphoteric oxide that reacts with strong acids and strong bases. 2+ ‐ (b)*BeO(s) + 2HCl(aq) + 3H2O(l) Be(H2O)4 (aq) + 2Cl (aq) BeO acting as a base 2+ + *BeO(s) + 2NaOH(aq) + H2O(l) Be(OH)4 (aq) + 2Na (aq) BeO acting as an acid 4) Describe the origins of sulfur dioxide that are causing environmental problems The oxidation of hydrogen sulfide (H2S) which is a product of bacterial decomposition 2H2S (g) + 2O2 (g) 2SO2 (g) + 2H2O (g) The burning of fossil fuels which usually contain sulfide minerals like FeS2. -

Acid Base and Salt
ACID BASE AND SALT • CLASSIFICATION BASED ON THEORIES/CONCEPTS • 1. ARRHENIUS CONCEPT OF ACID BASE • 2.BRONSTED LOWRY THEORY • 3. LEWIS THEORY • 4. LUX-FLOOD CONCEPT • 5. SOFT AND HARD ACID BASE THEORY ARRHENIUS CONCEPT • ACID PRODUCES • Base produces HYDROGEN ION IN Hydroxyl ion in aqueous AQUEOUS SOLUTION solution + - • HCl+H2O→H3O + Cl Or HCl(aq) → H O (aq) + Cl- (aq) NaOH(aq) → OH-(aq) + Na+(aq) • 3 • HI>HBr>HCl>HF(decreasing strength of acidity) KOH> NaOH> LiOH (Fajan’s concept) SiH + H O → SiO + H • 4 2 2 2 CH4 +H2O → no reaction Te(OH)6 > Si(OH)6 > B(OH)3 HF, H2O, NH3, CH4 Acidity?? DRAWBACKS • Unable to explain why NH3 which contains no OH- ions, is a • base and not an acid Arrange in order of increasing acidity: • Why a solution of FeCl3 is acidic 1.H3PO2, H3PO3, H3PO4 or why Na2S is alkaline • Limited to aqueous media only. 2. HClO , HClO , HClO , HOCl 4 3 2 Failed to explain why NaNH2 is 3. [Al(H O) ] +3 , [Fe(H O) ] +2 , [Co(H O) ] +2 alkaline and NH4Cl acidic in 2 6 2 6, 2 6 liquor ammonia • Failed to define inherent acid- base character(phenol/picric acid) Bronsted-Lowry Theory The Proton-donor-acceptor system • Bronsted Acids • Bronsted Bases • Molecular: HCl → H+ + Cl- • Molecular: H2O + H⁺ → H3O⁺ Cationic: [Al(H O) ] ⁺³ • 2 6 • Cationic: ↓ [Al(H O) (OH)] ⁺² [Al(H O) (OH)]⁺² + H⁺(proton) 2 5 2 5 • Anionic : HCO ¯ → H+ + CO ¯ Anionic: 3 3 CN- + H+ → HCN 2- - CO3 + H⁺ → H CO3 - HCl + H2O ↔ H3O⁺ + Cl Acid 1 Base 2 Acid 2 Base 1 (Conjugate acid-base pair) Reactions in aqueous media Effect of charge on acidity Comparison of -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M. -

An Efficient One-Pot Three-Component Synthesis of Novel Sulfanyl Tetrazoles Using Ionic Liquids
Hindawi Publishing Corporation Journal of Chemistry Volume 2013, Article ID 104690, 6 pages http://dx.doi.org/10.1155/2013/104690 Research Article An Efficient One-Pot Three-Component Synthesis of Novel Sulfanyl Tetrazoles Using Ionic Liquids Sankari Kanakaraju, Bethanamudi Prasanna, and G. V. P. Chandramouli Department of Chemistry, National Institute of Technology, Warangal 506 004, India Correspondence should be addressed to G. V. P. Chandramouli; [email protected] Received 13 June 2012; Accepted 11 August 2012 Academic Editor: Mohamed Afzal Pasha Copyright © 2013 Sankari Kanakaraju et al. is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. An efficient, simple, and environmentally benign method for the synthesis of novel sulfanyl tetrazoles has been achieved by one- pot three-component reaction of phenacyl bromides/3-(2-bromoacetyl)coumarins with KSCN and NaN using [Bmim]BF ionic liquid. It could be reused and recycled for four runs without signi�cant loss of product yield. 3 4 1. Introduction synthesize the compounds containing coumarin-substituted sulfanyl tetrazoles. Over the years, multicomponent reactions (MCRs) have e most convenient method of synthesizing tetrazoles become increasingly popular tools to ensure sufficient molec- is the addition of azide ions to nitriles. Earlier reported ular diversity and complexity. ey have gained signi�cant methods for the synthesis of 5-substituted tetrazoles suffer popularity in recent years due to their atom-economy and from drawbacks such as the use of strong Lewis acids, straightforward reaction design due to substantial minimiza- or expensive and toxic metals, and the in situ generated tion of waste, labour, time, and cost [1, 2]. -

Safe Generation and Synthetic Utilization of Hydrazoic Acid in a Continuous Flow Reactor
Full Paper Safe Generation and Synthetic Utilization of Hydrazoic Acid in a Continuous Flow Reactor Bernhard Gutmann1, David Obermayer1, Jean-Paul Roduit2, Dominique M. Roberge2* and C. Oliver Kappe1* 1Christian Doppler Laboratory for Microwave Chemistry and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, A-8010 Graz, Austria 2Microreactor Technology, Lonza AG, CH-3930 Visp, Switzerland Hydrazoic acid (HN3) was used in a safe and reliable way for the synthesis of 5-substitued-1H-tetrazoles and for the preparation of N-(2-azidoethyl)acylamides in a continuous flow format. Hydrazoic acid was generated in situ either from an aqueous feed of sodium azide upon mixing with acetic acid, or from neat trimethylsilyl azide upon mixing with methanol. For both processes, subsequent reaction of the in situ generated hydrazoic acid with either organic nitriles (tetrazole formation) or 2-oxazolines (ring opening to b-azido-carboxamides) was performed in a coil reactor in an elevated temperature/pressure regime. Despite the explosive properties of HN3, the reactions could be performed safely at very high temperatures to yield the desired products in short reaction times and in excellent product yields. The scalability of both protocols was demonstrated for selected examples. Employing a commercially available benchtop flow reactor, productivities of 18.9 g/h of 5-phenyltetrazole and 23.0 g/h of N-(1-azido-2-methylpropan- 2-yl)acetamide were achieved. Keywords: flow chemistry, hydrazoic acid, microreactor, process intensification, tetrazoles 1. Introduction a minimum [6–8]. The characteristics of microreaction technol- ogy (i.e., fast heat and mass transfer, and high pressure resis- Hydrazoic acid (HN ), a gas of “highly peculiar, dreadfully 3 tance of capillaries with small inner diameters) often allow pungent smell,” was discovered by Theodor Curtius in the 1890s temperatures to be used which would be unsafe in traditional by acidification of sodium azide [1].