Acidity of Elements in Periodic Table
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inorganic Chemistry Lesson 5 Oxides in Nature
Inorganic Chemistry Lesson 5 Oxides in nature. Acidic oxides. Acids. October 22, 2017 / 1 Oxides in nature. As we already know, oxygen is the most abun- dant element in the Earth crust: it constitutes about 49% of Earth lithosphere (by mass), and 20% of atmosphere (by volume).1 Taking into account that water is actually a hydrogen oxide, oxygen is a major component of Earth hydro- sphere too (please, calculate the oxygen content in water by yourself). Oxygen exists in a chemi- cally bound form everywhere except in the Earth atmosphere, and various oxides are among the most abundant forms of chemically bound oxy- gen on the Earth. Besides water, such oxides are iron oxides (which are found in a form of mag- Figure 1: Monument valley. Arizona and netite, hematite, goethite, limonite, etc, see Fig. Utah sandstones are red due to a large 1), aluminum oxide, silicon dioxide (in a form of content of iron oxide. quartz, opal etc). 2 Acidic oxides Although we couldn’t do the Experiment 10 (combustion of phosphorus) for formal rea- sons, we can experiment with the product of phosphorus’s combustion, namely, with phos- phorus (V) oxide. Let’s look at this compound closer. Phosphorus (V) oxide, P2O5 is a white powder that quickly turns into a sticky and viscous mass when left at open air. To understand why does it happen, let’s do an experiment. 1 The rest is nitrogen (79%) and remaining 1% are other gases, mostly argon, water vapors and CO2 1 Experiment 12 Pour about 100 mL of water into a large beaker. -

Zeolites 9 1.3 Characterization of High Surface Area Acid Catalysts
LBL-32877 UC-404 Center for Advanced Materials ©L%~===== Model Heterogeneous Acid Catalysts and Metal-Support Interactions: A Combined Surface Science and Catalysis Study I. Boszormenyi (Ph.D. Thesis) May 1991 --- I o..... ....0 r '1'10 n >- ~c::z.... CillO l'Dr+o I'D I'D ." ~[QO< --[Q - ....ttl Materials and Chemical Sciences Division a. IQ. U1 Lawrence Berkeley Laboratory 0 University of California I IS.) r r ttl ONE CYCLOTRON ROAD, BERKELEY, CA 94720 • (415) 486-4755 .... r D"O I '1 0 W 111'0 tv Prepared for the u.s. Department of Energy under Contract DE-AC03-76SF00098 '1'< (» '< -...J . tv -...J DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. Neither the United States Government nor any agency thereof, nor The Regents of the University of Califor nia, nor any of their employees, makes any warranty, express or im plied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe pri vately owned rights. Reference herein to any specific cornmercial product, process, or service by its trade name, trademark, manufac turer, or otherwise, does not necessarily constitute or imply its en dorsement, recommendation, or favoring by the United States Gov ernment or any agency thereof, or The Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or The Regents of the University of California and shall not be used for advertising or product endorsement pur poses. -

A Study of the Periodic Acid Oxidation of Cellulose Acetates of Low Acetyl
o A STUDY OF THE PERIODIC ACID OXIDATION OF CELLULOSE ACETATES OF LOW ACETYL CONTENT By Franklin Willard Herrick A THESIS Submitted to the School of Graduate Studies of Michigan State College of Agriculture and Applied Science in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Department of Chemistry 1950 ACOOTIBDGMENT Grateful recognition is given to Professor Bruce B. Hartsuch for his helpful guidance and inspiration throughout the course of this investigation. ********** ******** ****** **** ** * TABLE OF CONTENTS Page I INTRODUCTION.................................... ........ 1 The Structure of Cellulose. ..................... 1 The Present Problem.................................... 2 II GENERAL AMD HISTORICAL................................... 3 CELLULOSE ACETATE........................................ 3 PERIODATE OXIDATION OF CELLULOSE......................... 10 DISTRIBUTION OF HYDROXYL GROUPS IN CELLULOSE ACETATES.... 12 III EXPERIMENTAL............................................. 15 PREPARATION OF CELLULOSE ACETATE........................ 15 Materials.................................. .. ..... 15 Preparation of Standard Cellulose...................... 15 Preparation of Cellulose Acetates of Low Acetyl Content 16 Conditioning and Cutting of Standard Cellulose and Cellulose Acetate.................... 19 The Weighing of Linters ........................ 20 Analysis for Percentage of Combined Acetic Acid........ 21 Tabulation of Analyses of Cellulose Acetate Preparations 23 Calculation of the Degree -

IODINE Its Properties and Technical Applications
IODINE Its Properties and Technical Applications CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York IODINE Its Properties and Technical Applications ¡¡iiHiüíiüüiütitittüHiiUitítHiiiittiíU CHILEAN IODINE EDUCATIONAL BUREAU, INC. 120 Broadway, New York 5, New York 1951 Copyright, 1951, by Chilean Iodine Educational Bureau, Inc. Printed in U.S.A. Contents Page Foreword v I—Chemistry of Iodine and Its Compounds 1 A Short History of Iodine 1 The Occurrence and Production of Iodine ....... 3 The Properties of Iodine 4 Solid Iodine 4 Liquid Iodine 5 Iodine Vapor and Gas 6 Chemical Properties 6 Inorganic Compounds of Iodine 8 Compounds of Electropositive Iodine 8 Compounds with Other Halogens 8 The Polyhalides 9 Hydrogen Iodide 1,0 Inorganic Iodides 10 Physical Properties 10 Chemical Properties 12 Complex Iodides .13 The Oxides of Iodine . 14 Iodic Acid and the Iodates 15 Periodic Acid and the Periodates 15 Reactions of Iodine and Its Inorganic Compounds With Organic Compounds 17 Iodine . 17 Iodine Halides 18 Hydrogen Iodide 19 Inorganic Iodides 19 Periodic and Iodic Acids 21 The Organic Iodo Compounds 22 Organic Compounds of Polyvalent Iodine 25 The lodoso Compounds 25 The Iodoxy Compounds 26 The Iodyl Compounds 26 The Iodonium Salts 27 Heterocyclic Iodine Compounds 30 Bibliography 31 II—Applications of Iodine and Its Compounds 35 Iodine in Organic Chemistry 35 Iodine and Its Compounds at Catalysts 35 Exchange Catalysis 35 Halogenation 38 Isomerization 38 Dehydration 39 III Page Acylation 41 Carbón Monoxide (and Nitric Oxide) Additions ... 42 Reactions with Oxygen 42 Homogeneous Pyrolysis 43 Iodine as an Inhibitor 44 Other Applications 44 Iodine and Its Compounds as Process Reagents ... -

Acid Base Notes Notes.Notebook March 31, 2015
Acid Base Notes Notes.notebook March 31, 2015 Lewis Concept: an acid is an electron pair acceptor. A base is an electron pair donor. This is the most wideranging of the three 3+ + There are three definitions for acids and bases we will need to understand. (i.e. it works for everything). Examples of Lewis acids include Al , H , BF3. Examples of Lewis bases include NO2 , NH3, and H2O. Arrhenius Concept: an acid supplies H+ to an aqueous solution. A base supplies OH to an aqueous solution. This is the oldest definition but most limiting. Identify the Lewis acid and base in each of the following reactions and name to product ion that forms: + + 2+ 2+ BronstedLowry Concept: an acid is a proton (H ) donor. A base is a proton (H ) acceptor. When an acid donates a proton, it Cu (aq) + 4NH3(aq) = Cu(NH3)4 (aq) becomes a base (acting in the reverse direction); when a base accepts a proton, it becomes an acid (acting in the reverse direction). You will need to identify conjugate acidbase pairs. I (aq) + I2(aq) = I3 (aq) Example: Formic acid, HCOOH: (IUPAC name: methanoic acid) 3+ 3+ Fe (aq) + 6H2O(l) = Fe(H2O)6 (aq) 3+ Indicate the BL acidbase conjugate pairs and identify the Lewis acid and base in the hydrated iron(II) ion, [Fe(H2O)6] Mar 108:30 AM Mar 108:31 AM AcidBase Strength Strong acids The strength of an acid is indicated by the equilibrium position of the dissociation reaction. -

Nitrous Acid)
5. Nitrogen Group Content 5.1 Occurrence 5.2 Group Properties Group 5.3 Physical Properties 15 or VA 5.4 Syntheses 7 1772 5.5 Chemical Behaviour N 15 5.6 Applications 1669 5.7 Chemistry of Elemental Nitrogen P 33 5.8 Compounds Made of Nitrogen and Hydrogen Antique 5.9 Nitrogen Compounds with Oxygen As 51 5.10 Nitrogen Compounds with Halides Antique Sb 5.11 Phosphorus/Hydrogen Compounds 83 1753 5.12 Phosphorus Oxides Bi 5.13 Oxo Acids of Phosphorus 115 2003 5.14 Phosphorus Compounds with Halides Mc 5.15 Arsenic, Antimony and Bismuth 5.16 Biological Aspects „Penteles“ Inorganic Chemistry I Slide 1 Prof. Dr. T. Jüstel 5.1 Occurrence Außer Phosphor kommen alle Pentele auch elementar (gediegen) vor Nitrogen (nitrogenium) N2 (78.1% in the air) NaNO3 Chile saltpetre KNO3 Saltpetre Phosphorus (phosphoros) Ca5(PO4)3(OH,F) Apatite greek: lightbearer Ca3(PO4)2 Phosphorite . Fe3(PO4)2 8H2O Vivianite Arsenic (arsenikos) FeAsS Arsenopyrite greek: mineral name As4S4 Realgar As4S3 Antimony (antimonium) Sb native Stibium = greek mineral name Sb2S3 Bismuth (bismutum) Bi native german: Wismut = Mutung “in the meadows” Bi2S3 Inorganic Chemistry I Slide 2 Prof. Dr. T. Jüstel 5.2 Group Properties Whereas Nitrogen Exhibits the Typical Properties of A Non-Metal, Bismuth Is Solely Metallic N P As Sb Bi Atomic number 7 15 33 51 83 Electronic [He] [Ne] [Ar] [Kr] [Xe]4f14 configuration 2s22p3 3s23p3 3d104s24p3 4d105s25p3 5d106s26p3 Electronegativity 3.0 2.1 2.2 1.8 1.7 Ionisation energy [eV] 14.5 11.0 9.8 8.6 7.3 Electronic affinity [eV] -0.3 0.6 0.7 0.6 > 0.7 Character of oxides acidic acidic amphoteric amphoteric alkaline Oxidation states -3, ...…, +5 With increasing atomic number, the oxidation state +3 becomes more stable, whilst the oxidation state +5 becomes instable. -

Synthesis, Characterization, and Application of Zirconia and Sulfated Zirconia Derived from Single Source Precursors
SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS By MOHAMMED H. AL-HAZMI Bachelor of Science King Saud University Riyadh, Saudi Arabia 1995 Master of Science King Saud University Riyadh, Saudi Arabia 1999 Submitted to the Faculty of the Graduate College of the Oklahoma State University In partial fulfilment of The requirements for The Degree of DOCTOR OF PHILOSOPHY May, 2005 SYNTHESIS, CHARACTERIZATION, AND APPLICATION OF ZIRCONIA AND SULFATED ZIRCONIA DERIVED FROM SINGLE SOURCE PRECURSORS Thesis Approved: _______________Dr. Allen Apblett_____________ Thesis Adviser ___________________________________________ ______________Dr. K. Darrell Berlin___________ _____________Dr. LeGrand Slaughter__________ _______________Dr. Gary Foutch______________ _____________Dr. A. Gordon Emslie___________ Dean of the Graduate College ii ACKNOWLEDGMENTS The words are inadequate to express my truthful and profound thanks to my phenomenal advisor Dr. Allen W. Apblett for his advice and guidance, continued support, tremendous help, encouragements, and insight and sharp criticism. Since the time that he offered and accepted me to work in this intriguing project, and during the last four and half years, I have learned lots of things from his way of thinking and his research methodology. When encountering some problems and difficulties in different research issues, he simply gives the guidance and the strength to embellish an acceptable idea into a great one. I can honestly say that this Ph.D. dissertation work would not be accomplished without his outstanding supervision, scientific knowledge and experience, and his magnanimous and warm personality of research. My committee members, Dr. Berlin, Dr. Slaughter, and Dr. Foutch are deeply appreciated for their assistance, reading, editing, and invaluable discussion and comments. -

Information to Users
INFORMATION TO USERS While the most advanced technology has been used to photograph and reproduce this manuscript, the quality of the reproduction is heavily dependent upon the quality of the material submitted. For example: • Manuscript pages may have indistinct print. In such cases, the best available copy has been filmed. • Manuscripts may not always be complete. In such cases, a note will indicate that it is not possible to obtain missing pages. • Copyrighted material may have been removed from the manuscript. In such cases, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, and charts) are photographed by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each oversize page is also filmed as one exposure and is available, for an additional charge, as a standard 35mm slide or as a 17”x 23” black and white photographic print. Most photographs reproduce acceptably on positive microfilm or microfiche but lack the clarity on xerographic copies made from the microfilm. For an additional charge, 35mm slides of 6”x 9” black and white photographic prints are available for any photographs or illustrations that cannot be reproduced satisfactorily by xerography. 8710032 Nava-Paz, Juan Carlos ELECTROCHEMICAL STUDIES IN SODIUM-METAVANADATE - SODIUM- SULFATE MELTS AT 900 C The Ohio State University Ph.D. 1987 University Microfilms International300 N. Zeeb Road, Ann Arbor, Ml 48106 Copyright 1987 by Nava-Paz, Juan Carlos All Rights Reserved PLEASE NOTE: In all cases this material has been filmed in the best possible way from the available copy. -
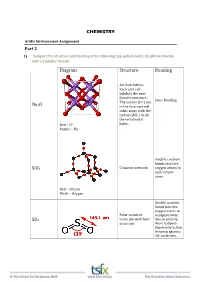
CHEMISTRY Diagram Structure Bonding Na2o Sio2
CHEMISTRY Acidic Environment Assignment Part 2 1) Compare the structure and bonding of the following: (a) sodium oxide, (b) silicon dioxide, and (c) sulphur dioxide Diagram Structure Bonding An ionic lattice. Each unit cell exhibits the anti‐ fluorite structure. Ionic bonding The anions (O2‐) are Na2O in the face‐centred cubic array with the cations (Na+) in all the tetrahedral Red – O2‐ holes. Purple – Na+ Double covalent bonds join two SiO2 Covalent network oxygen atoms to each silicon atom. Red – Silicon Black – Oxygen Double covalent bonds join two oxygen atoms to Polar covalent a sulphur atom. SO2 molecule with bent Due to polarity, structure. there is dipole‐ dipole interaction between gaseous SO2 molecules. 2) Write equations for the reaction of the following with water: COMPOUND REACTION WITH WATER Carbon dioxide CO2(g) + H2O(l) H2CO3(aq) Sodium oxide Na2O(aq) + H2O(l) 2NaOH(aq) Calcium oxide CaO(aq) + H2O(l) Ca(OH)2(aq) Sulfur dioxide SO2(g) + H2O(l) H2SO3(aq) Sulfur trioxide SO3(g) + H2O(l) H2SO4(aq) Nitrogen dioxide NO2(g) + H2O(l) HNO2(aq) + HNO3(aq) 3) Beryllium oxide is amphoteric. (a) Explain what is meant by amphoteric, and (b) Study the two equations below*. Balance them, and indicate whether BeO is acting as an acid or base (a) ‘Amphoteric’ is a term used to describe a substance that exhibits both acidic and basic properties. Beryllium oxide is an amphoteric oxide that reacts with strong acids and strong bases. 2+ ‐ (b)*BeO(s) + 2HCl(aq) + 3H2O(l) Be(H2O)4 (aq) + 2Cl (aq) BeO acting as a base 2+ + *BeO(s) + 2NaOH(aq) + H2O(l) Be(OH)4 (aq) + 2Na (aq) BeO acting as an acid 4) Describe the origins of sulfur dioxide that are causing environmental problems The oxidation of hydrogen sulfide (H2S) which is a product of bacterial decomposition 2H2S (g) + 2O2 (g) 2SO2 (g) + 2H2O (g) The burning of fossil fuels which usually contain sulfide minerals like FeS2. -

Acid Base and Salt
ACID BASE AND SALT • CLASSIFICATION BASED ON THEORIES/CONCEPTS • 1. ARRHENIUS CONCEPT OF ACID BASE • 2.BRONSTED LOWRY THEORY • 3. LEWIS THEORY • 4. LUX-FLOOD CONCEPT • 5. SOFT AND HARD ACID BASE THEORY ARRHENIUS CONCEPT • ACID PRODUCES • Base produces HYDROGEN ION IN Hydroxyl ion in aqueous AQUEOUS SOLUTION solution + - • HCl+H2O→H3O + Cl Or HCl(aq) → H O (aq) + Cl- (aq) NaOH(aq) → OH-(aq) + Na+(aq) • 3 • HI>HBr>HCl>HF(decreasing strength of acidity) KOH> NaOH> LiOH (Fajan’s concept) SiH + H O → SiO + H • 4 2 2 2 CH4 +H2O → no reaction Te(OH)6 > Si(OH)6 > B(OH)3 HF, H2O, NH3, CH4 Acidity?? DRAWBACKS • Unable to explain why NH3 which contains no OH- ions, is a • base and not an acid Arrange in order of increasing acidity: • Why a solution of FeCl3 is acidic 1.H3PO2, H3PO3, H3PO4 or why Na2S is alkaline • Limited to aqueous media only. 2. HClO , HClO , HClO , HOCl 4 3 2 Failed to explain why NaNH2 is 3. [Al(H O) ] +3 , [Fe(H O) ] +2 , [Co(H O) ] +2 alkaline and NH4Cl acidic in 2 6 2 6, 2 6 liquor ammonia • Failed to define inherent acid- base character(phenol/picric acid) Bronsted-Lowry Theory The Proton-donor-acceptor system • Bronsted Acids • Bronsted Bases • Molecular: HCl → H+ + Cl- • Molecular: H2O + H⁺ → H3O⁺ Cationic: [Al(H O) ] ⁺³ • 2 6 • Cationic: ↓ [Al(H O) (OH)] ⁺² [Al(H O) (OH)]⁺² + H⁺(proton) 2 5 2 5 • Anionic : HCO ¯ → H+ + CO ¯ Anionic: 3 3 CN- + H+ → HCN 2- - CO3 + H⁺ → H CO3 - HCl + H2O ↔ H3O⁺ + Cl Acid 1 Base 2 Acid 2 Base 1 (Conjugate acid-base pair) Reactions in aqueous media Effect of charge on acidity Comparison of -

Kinetics Op Oxidation Op Some Organic Compounds By
KINETICS OP OXIDATION OP SOME ORGANIC COMPOUNDS BY PERIODIC ACID, STUDIED BY ULTRA-VIOLET SPECTROSCOPY by ALI M KHALIPA A thesis submitted to the University of Surrey for the degree of Doctor of Philosophy, Cecil Davies Laboratory Department of Chemistry University of Surrey Guildford January,1982 ProQuest Number: 10800213 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10800213 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 DEDICATION TO MY DEAR PARENTS ACKNOWLEDGEMENTS I am greatly indebted to my supervisor, Dr.G.J.Buist, for his invaluable guidance,help,enthusiasm and advice through the period of this research work. I am also grateful to many of my friends and colleagues of the Department of Chemistry, University of Surrey, who have made my stay in England enjoyable, and helped in various aspects of the research. Finally, I would like to express my most sincere appreciation to the Iraqi Ministry of Higher Education and Scientific Research for the scholarship which financed the major part of my study and to the Chemistry Department at the University of Surrey for the very generous use of departmental facilities. -

I. Ionization and Hydration Equilibria of Periodic Acid; II. Solubility and Complex Ion Formation of the Rare Earth Oxalates Carl E
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1950 I. Ionization and hydration equilibria of periodic acid; II. Solubility and complex ion formation of the rare earth oxalates Carl E. Crouthamel Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Inorganic Chemistry Commons Recommended Citation Crouthamel, Carl E., "I. Ionization and hydration equilibria of periodic acid; II. Solubility and complex ion formation of the rare earth oxalates" (1950). Retrospective Theses and Dissertations. 14235. https://lib.dr.iastate.edu/rtd/14235 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS This manuscript has been reproduced from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are In typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion.