Morphometric and Topographic Study of Foramen Ovale in Indian Skulls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Results Description of the SKULLS. the Overall Size of Both Skulls Was Considered to Be Within Normal Limits for Their Ethnic
Ossification Defects and Craniofacial Morphology In Incomplete Forms of Mandibulofacial Dysostosis A Description of Two Dry Skulls ERIK DAHL, D.D.S., DR. ODONT. ARNE BJORK, D.D.S., ODONT. DR. Copenhagen, Denmark The morphology of two East Indian dry skulls exhibiting anomalies which were suggested to represent incomplete forms of mandibulofacial dysostosis is described. Obvious although minor ossification anomalies were found localized to the temporal, sphenoid, the zygomatic, the maxillary and the mandibular bones. The observations substantiate the concept of the regional and bilateral nature of this malformation syndrome. Bilateral orbital deviations, hypoplasia of the malar bones, and incomplete zygomatic arches appear to be hard tissue aberrations which may be helpful in exami- nation for subclinical carrier status. Changes in mandibular morphology seem to be less distinguishing features in incomplete or abortive types of mandibulofacial dysostosis. KEY WORDS craniofacial problems, mandible, mandibulofacial dysostosis, maxilla, sphenoid bone, temporal bone, zygomatic bone Mandibulofacial dysostosis (MFD) often roentgencephalometric examinations were results in the development of a characteristic made of the skulls, and tomograms were ob- facial disfigurement with considerable simi- tained of the internal and middle ear. Com- larity between affected individuals. However, parisons were made with normal adult skulls the symptoms may vary highly in respect to and with an adult skull exhibiting the char- type and degree, and both incomplete and acteristics of MFD. All of the skulls were from abortive forms of the syndrome have been the same ethnic group. ' reported in the literature (Franceschetti and Klein, 1949; Moss et al., 1964; Rogers, 1964). Results In previous papers, we have shown the DEsCRIPTION OF THE SKULLS. -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -
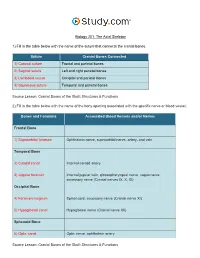
The Axial Skeleton Visual Worksheet
Biology 201: The Axial Skeleton 1) Fill in the table below with the name of the suture that connects the cranial bones. Suture Cranial Bones Connected 1) Coronal suture Frontal and parietal bones 2) Sagittal suture Left and right parietal bones 3) Lambdoid suture Occipital and parietal bones 4) Squamous suture Temporal and parietal bones Source Lesson: Cranial Bones of the Skull: Structures & Functions 2) Fill in the table below with the name of the bony opening associated with the specific nerve or blood vessel. Bones and Foramina Associated Blood Vessels and/or Nerves Frontal Bone 1) Supraorbital foramen Ophthalmic nerve, supraorbital nerve, artery, and vein Temporal Bone 2) Carotid canal Internal carotid artery 3) Jugular foramen Internal jugular vein, glossopharyngeal nerve, vagus nerve, accessory nerve (Cranial nerves IX, X, XI) Occipital Bone 4) Foramen magnum Spinal cord, accessory nerve (Cranial nerve XI) 5) Hypoglossal canal Hypoglossal nerve (Cranial nerve XII) Sphenoid Bone 6) Optic canal Optic nerve, ophthalmic artery Source Lesson: Cranial Bones of the Skull: Structures & Functions 3) Label the anterior view of the skull below with its correct feature. Frontal bone Palatine bone Ethmoid bone Nasal septum: Perpendicular plate of ethmoid bone Sphenoid bone Inferior orbital fissure Inferior nasal concha Maxilla Orbit Vomer bone Supraorbital margin Alveolar process of maxilla Middle nasal concha Inferior nasal concha Coronal suture Mandible Glabella Mental foramen Nasal bone Parietal bone Supraorbital foramen Orbital canal Temporal bone Lacrimal bone Orbit Alveolar process of mandible Superior orbital fissure Zygomatic bone Infraorbital foramen Source Lesson: Facial Bones of the Skull: Structures & Functions 4) Label the right lateral view of the skull below with its correct feature. -

Orbital Meningiomas Meningiomas Orbitários Carlos Eduardo Da Silva, M.D
31 Revisão Orbital Meningiomas Meningiomas Orbitários Carlos Eduardo da Silva, M.D. 1 Paulo Eduardo Freitas, M.D. Ph.D.2 Alicia Del Carmem Becerra Romero, M.D.3 Tâmen Moyses Pereyra4 Vicente Faraon Fonseca4 Willian Alves Martins4 Márcio Aloisio Bezerra Cavalcanti Rockenbach4 Fáberson João Mocelin Oliveira4 ABSTRACT RESUMO Orbital meningiomas usually invade the orbit as an extension Meningeomas orbitários invadem a órbita, na maioria dos ca- of the sphenoid wing meningiomas, clinoidal meningiomas, sos, como uma extensão de meningeomas da asa do esfenóide, cavernous sinus meningiomas and tuberculum sella tumors. meningeomas do seio cavernoso, meningeomas da clinóide e They also arise into the orbit originating from the optic sheath do tubérculo da sela. Eles também podem ser originados do or as ectopical lesions. The authors present a review of clini- revestimento dural do nervo óptico ou como lesões ectópicas cal aspects and surgical treatment of the orbital meningio- intraorbitais. Os autores apresentam uma revisão dos aspectos mas. Material and methods: The authors present a literature clínicos e cirúrgicos dos meningeomas orbitários. Material e review of the anatomical, clinical, and surgical aspects of métodos: Os autores apresentam uma revisão da literatura dos the orbital meningiomas, add illustrative cases, pointing aspectos anatômicos, clínicos e cirúrgicos dos meningeomas their principal concerns about the treatment of such tumors. orbitários, com casos ilustrativos, apresentando suas principais Results: Exophthalmos and unilateral visual loss are the most preocupações no manejo destes tumores. Resultados: Exoftal- common features of the orbital meningiomas. There are two mia e perda visual unilateral são os achados mais frequentes important surgical routes to approach such tumors, which are nos meningeomas orbitários. -

Topographical Anatomy and Measurements of Selected Parameters of the Rat Temporal Bone
Folia Morphol. Vol. 67, No. 2, pp. 111–119 Copyright © 2008 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Topographical anatomy and measurements of selected parameters of the rat temporal bone J. Wysocki Clinic of Otolaryngology, Medical Faculty No. 2, Medical University of Warsaw, Kajetany, Poland Institute of Physiology and Pathology of Hearing, Warsaw, Poland [Received 22 October 2008; Accepted 22 November 2008] On the basis of dissection of 24 bones of 12 black rats a systematic anatomical description was made and measurements of selected size parameters of the tem- poral bone were taken. Besides the main air space in the middle ear, the tym- panic bulla, there are also additional air cells, namely the anterior and posterior epitympanic recesses, containing the head of the malleus and the body of the incus. On the side of the epitympanic recesses the following are easily accessi- ble: the malleus head and the core of the incus, the superior and lateral semicir- cular canals and the facial nerve. On the side of the ventral tympanic bulla it is easy access to both windows and the cochlea. The semicircular canals are rela- tively large, the lateral canal being the largest and the posterior the smallest. The length of the spiral canal of the cochlea does not exceed 11 mm. It is worth mentioning that both the vertical and horizontal dimensions of the scala vestibuli and scala tympani do not even exceed 0.7 mm in the basal turn, and are signif- icantly decreased to tenths of a millimetre in further turns. -

The Importance of Shaving the Zygomatic Process During Reduction Malarplasty
Int. J. Oral Maxillofac. Surg. 2016; 45: 1002–1005 http://dx.doi.org/10.1016/j.ijom.2016.01.001, available online at http://www.sciencedirect.com Technical Note Cosmetic Surgery T. S. Lee Centre for Maxillofacial Surgery, ID Hospital, The importance of shaving the Seoul, Republic of Korea zygomatic process during reduction malarplasty T.S. Lee: The importance of shaving the zygomatic process during reduction malarplasty. Int. J. Oral Maxillofac. Surg. 2016; 45: 1002–1005. # 2016 International Association of Oral and Maxillofacial Surgeons. Published by Elsevier Ltd. All rights reserved. Abstract. Reduction malarplasty surgery has become increasingly popular in recent years, especially in many East Asian countries. This is, in part, because many Asians consider a small, smooth, and feminine face to be more attractive and aesthetically desirable. Among the various reduction malarplasty methods, the L-shaped osteotomy technique, through intraoral and sideburn incisions, is now one of the most frequently performed surgical techniques. During the surgical procedure, it is important to shave the zygomatic process of the temporal bone through the sideburn incision. By carrying out this simple adjunctive procedure, several remarkable results can be achieved. The facial width is reduced, especially in those patients with Key words: reduction malarplasty; facial bone protrusion of the posterior portion of the arch. The outward curvature of the contouring; zygoma reduction. zygomatic arch is changed to point inward. And finally, the bony step that originates from the medial repositioning of the zygomatic arch is reduced, resulting in Accepted for publication 5 January 2016 decreased palpability. Available online 22 January 2016 The zygomatic bone plays an important Ever since Onizuka et al. -

Orbitozygomatic Craniotomy in Three Pieces
DOI: 10.1590/0004-282X20160024 TRICK OF THE TRADE Orbitozygomatic craniotomy in three pieces: tips and tricks Craniotomia orbitozigomática em três peças: dicas e truques Feres Chaddad-Neto1,2, Hugo Leonardo Doria-Netto1,2, José Maria Campos-Filho1,2, Mateus Reghin-Neto2, Marcos Devanir Silva-Costa1, Evandro Oliveira2,3 ABSTRacT Objective: Didactically describe the orbitozygomatic craniotomy made in three pieces. Method: This approach was performed, from 2002 to 2011, in 49 patients admitted at Beneficência Portuguesa of São Paulo Hospital.Results: Twenty-seven patients had vascular lesions and twenty-two suffered for intracranial skull base tumors. The vascular lesions varied from cavernous angiomas inside the mesencephalum, high bifurcation basilar tip aneurysms, superior cerebellar arteries aneurysms and arteriovenous malformations in the interpeduncular cistern. Skull base tumors as meningiomas, interpeduncular hamartomas and third ventricle floor gliomas were among the neoplastic lesions approached. We had no permanent injuries and minimal transient complications had occurred. Conclusion: It is a descriptive text, organized in the sequence of the main stages in which such a craniotomy is performed, describing in details the technique in which this group of evolutionarily authors came to accomplish the task. Keywords: orbitozygomatic, craniotomy, neurosurgery, microsurgery. RESUMO Objetivo: Descrever didaticamente a craniotomia orbitozigomática realizada em três peças. Método: Esse acesso foi realizado em 49 pacientes, de 2002 a 2011 em pacientes admitidos no Hospital Beneficência Portuguesa de São Paulo.Resultados: Vinte e sete pacientes apresentavam lesões vasculares e vinte e dois sofriam de tumores da base do crânio. As lesões vasculares variaram entre angiomas cavernosos do mesencéfalo, aneurismas topo da artéria basilar com bifurcações altas, aneurismas da artéria cerebelas superior a malformações arteriovenosas na cisterna interpeduncular. -

Skeletal System + Joints)
Chapters 610 (Skeletal system + joints) Exercise 6: Introduction to the Skeletal System. Figure 6.1 Axial or Appendicular (Divisions) Figure 6.2 Major bones in the body Figure 6.3 Bone “Classification” Figure 6.5‐6.6 Bone model and slides (parts) Table 6.2 Terminology Exercise 7: Appendicular Skeleton Scapula: o Acromion process o Scapular notch o Coracoid process o Supraspinous fossa o Glenoid Cavity o Spine o Lateral (axillary) border o Subscapular fossa o Inferior angle o Medial boarder o Superior angle o Infraspinous fossa o Superior boarder Clavicle o Coronoid turbercle o Sternal end o Acromial end Humerous o Head o Olecranon fossa o Greater turbercle o Lateral epicondyle o Lesser tubercle o Medial epicondyle o Deltoid tuberosity o Capitulum o Radial fossa o Trochlea o Coronoid fossa Radius o Head o Ulnar notch o Neck o Styloid process o Radial tuberosity Ulna o Olecranon process o Radial notch o Trochlear notch o Head of the ulna o Coronoid process o Styloid process Hand o Carpals o Metacarpals o Phalanges Pelvic girdle (7.6 and 7.7) o Sacrum o Coxal bone o Sacrolilliac joint . Illium o Acetabulum . Pubis o Symphysis pubis . Ischium o Obturator foramen o Ischial tuberosity o Iliac crest o Ischial ramus o Illiac fossa Femur o Head o Fovea capitis o Greater trochanter o Body o Neck o Lateral epicondyle o Intertrochanteric line o Medial epicondyle (anterior) o Patellar groove o Intertrochanteric crest o Medial condyle (posterior) o Lateral condyle o Lesser trochanter o Intercondylar fossa Patella o Base o Apex Tibia -

Laboratory 1 Worksheet: the Skull Objective: Learn About The
Skull_Skeleton_Lab3.doc 09/15/09 page 1 of 48 Laboratory 1 Worksheet: The Skull Objective: Learn about the mammalian skull, and be able to define and/or identify on a specimen all underlined terms. Assignment: Turn in two photos/drawings of a skull with bones and structures labeled. Use a skull of a coyote (Canis latrans) or red fox (Vulpes vulpes) and identify the following bones and other features. Canids have a fairly "primitive" skull large enough to identify different bones. For comparative purposes other skulls are shown in the figures to illustrate differences among groups or features missing from the canid skull. After identifying features on a canid skull, you should be able to find the same bones or features on skulls of other mammals. Main features of mammal skulls are the zygomatic arches (the bars on both sides of the skull) under which the jaw muscles reach from the lower jaw to the back of the head, a secondary palate that separates the mouth from the nasal passages, and a mandible (lower jaw) consisting of a single dentary bone on the left and right sides. Cranium The cranium is divided into two regions: braincase and rostrum. The braincase, more developed in mammals than in other vertebrates, contains the brain. The rostrum corresponds to the snout or muzzle. Dorsal aspect of the cranium.—Bones seen from a dorsal aspect on a canid skull are shown in Fig. 3. The nasal bones are paired bones forming a “roof” over the nasal passages. The paired premaxillary bones form the lower margin of the nasal openings (nares) and the anteriormost part of the bony palate at the anterior upper jaw. -

Surgical Anatomy of the Temporal Bone Gülay Açar and Aynur Emine Çiçekcibaşı
Chapter Surgical Anatomy of the Temporal Bone Gülay Açar and Aynur Emine Çiçekcibaşı Abstract Numerous neurological lesions and tumors of the paranasal sinuses and oral cavity may spread into the middle and posterior cranial fossae through the ana- tomical apertures. For the appropriate management of these pathologies, many extensive surgical approaches with a comprehensive overview of the anatomical landmarks are required from the maxillofacial surgery’s point of view. The surgical significance lies in the fact that iatrogenic injury to the petrous segment of the tem- poral bone including the carotid artery, sigmoid sinus, and internal jugular vein, can lead to surgical morbidity and postoperative pseudoaneurysm, vasospasm, or carotid-cavernous fistula. To simplify understanding complex anatomy of the temporal bone, we aimed to review the surgical anatomy of the temporal bone focusing on the associations between the surface landmarks and inner structures. Also, breaking down an intricate bony structure into smaller parts by compart- mental approach could ease a deep concentration and navigation. To identify the anatomic architecture of the temporal bone by using reference points, lines and compartments can be used to supplement anatomy knowledge of maxillofacial surgeons and may improve confidence by surgical trainees. Especially, this system- atic method may provide an easier way to teach and learn surgical spatial structure of the petrous pyramid in clinical applications. Keywords: maxillofacial surgery, segmentation, surface landmarks, surgical anatomy, temporal bone 1. Introduction The temporal bone is a dense complex bone that constitutes the lower lateral aspect of the skull and has complex anatomy because of the three-dimensional relationships between neurovascular structures. -

Temporomandibular Joint (TMJ)
Regions of the Head 9. Oral Cavity & Perioral Regions Temporomandibular Joint (TMJ) Zygomatic process of temporal bone Articular tubercle Mandibular Petrotympanic fossa of TMJ fissure Postglenoid tubercle Styloid process External acoustic meatus Mastoid process (auditory canal) Atlanto- occipital joint Fig. 9.27 Mandibular fossa of the TMJ tubercle. Unlike other articular surfaces, the mandibular fossa is cov- Inferior view of skull base. The head (condyle) of the mandible artic- ered by fi brocartilage, not hyaline cartilage. As a result, it is not as ulates with the mandibular fossa of the temporal bone via an articu- clearly delineated on the skull (compare to the atlanto-occipital joints). lar disk. The mandibular fossa is a depression in the squamous part of The external auditory canal lies just posterior to the mandibular fossa. the temporal bone, bounded by an articular tubercle and a postglenoid Trauma to the mandible may damage the auditory canal. Head (condyle) of mandible Pterygoid Joint fovea capsule Neck of Coronoid mandible Lateral process ligament Neck of mandible Lingula Mandibular Stylomandibular foramen ligament Mylohyoid groove AB Fig. 9.28 Head of the mandible in the TMJ Fig. 9.29 Ligaments of the lateral TMJ A Anterior view. B Posterior view. The head (condyle) of the mandible is Left lateral view. The TMJ is surrounded by a relatively lax capsule that markedly smaller than the mandibular fossa and has a cylindrical shape. permits physiological dislocation during jaw opening. The joint is stabi- Both factors increase the mobility of the mandibular head, allowing lized by three ligaments: lateral, stylomandibular, and sphenomandib- rotational movements about a vertical axis.