150259-ID-None.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
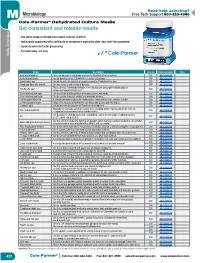
Get Consistent and Reliable Results
Need help selecting? M Microbiology Free Tech Support 800-323-4340 Cole-Parmer® Dehydrated Culture Media Get consistent and reliable results – Use when ready or dehydrated culture extends shelf life – Individually numbered with certificate of compliance expiration date, and shelf-life guarantee – Quick dissolve for faster processing – For laboratory use only Media, Dehydrated Media, Media Description Size (g) Catalog number Price Agar, bacteriological This clear gel agar is high grade and manufactured using the ice method 500 GH-14200-10 Azide dextrose broth Use for detection of fecal Streptococci in water and sewage 500 GH-14202-00 Baird parker agar Use for the selective isolation of coagulase positive Staphylococci in food 500 GH-14202-01 Blood agar base with low pH Use to cultivate a wide variety of bacteria 500 GH-14202-02 This is used as a differential medium in the isolation and presumptive identification of Bile Esculin agar 500 GH-14202-03 enterococci/group D streptococci Brain heart infusion agar Use to cultivate a wide spectrum of bacteria, yeasts, and molds 500 GH-14200-12 Brain heart infusion broth Use to cultivate fastidious aerobic and anaerobic bacteria 500 GH-14202-05 Brilliant green bile broth A standard methods medium for confirmed and completed tests for coliforms in water 500 GH-14200-14 Buffered peptone water Helps in the recovery of Salmonella from foods after preservation techniques 500 GH-14202-08 Cetrimide agar Use for the selective isolation of Pseudomonas aeruginosa 500 GH-14200-52 This broth is especially suited for environmental sampling where neutralization of the chemical D/E neutralizing Broth 500 GH-14202-11 is important to determining the bactericidal activity Use to isolate E. -

Prepared Culture Media
PREPARED CULTURE MEDIA 121517SS PREPARED CULTURE MEDIA Made in the USA AnaeroGRO™ DuoPak A 02 Bovine Blood Agar, 5%, with Esculin 13 AnaeroGRO™ DuoPak B 02 Bovine Blood Agar, 5%, with Esculin/ AnaeroGRO™ BBE Agar 03 MacConkey Biplate 13 AnaeroGRO™ BBE/PEA 03 Bovine Selective Strep Agar 13 AnaeroGRO™ Brucella Agar 03 Brucella Agar with 5% Sheep Blood, Hemin, AnaeroGRO™ Campylobacter and Vitamin K 13 Selective Agar 03 Brucella Broth with 15% Glycerol 13 AnaeroGRO™ CCFA 03 Brucella with H and K/LKV Biplate 14 AnaeroGRO™ Egg Yolk Agar, Modified 03 Buffered Peptone Water 14 AnaeroGRO™ LKV Agar 03 Buffered Peptone Water with 1% AnaeroGRO™ PEA 03 Tween® 20 14 AnaeroGRO™ MultiPak A 04 Buffered NaCl Peptone EP, USP 14 AnaeroGRO™ MultiPak B 04 Butterfield’s Phosphate Buffer 14 AnaeroGRO™ Chopped Meat Broth 05 Campy Cefex Agar, Modified 14 AnaeroGRO™ Chopped Meat Campy CVA Agar 14 Carbohydrate Broth 05 Campy FDA Agar 14 AnaeroGRO™ Chopped Meat Campy, Blood Free, Karmali Agar 14 Glucose Broth 05 Cetrimide Select Agar, USP 14 AnaeroGRO™ Thioglycollate with Hemin and CET/MAC/VJ Triplate 14 Vitamin K (H and K), without Indicator 05 CGB Agar for Cryptococcus 14 Anaerobic PEA 08 Chocolate Agar 15 Baird-Parker Agar 08 Chocolate/Martin Lewis with Barney Miller Medium 08 Lincomycin Biplate 15 BBE Agar 08 CompactDry™ SL 16 BBE Agar/PEA Agar 08 CompactDry™ LS 16 BBE/LKV Biplate 09 CompactDry™ TC 17 BCSA 09 CompactDry™ EC 17 BCYE Agar 09 CompactDry™ YMR 17 BCYE Selective Agar with CAV 09 CompactDry™ ETB 17 BCYE Selective Agar with CCVC 09 CompactDry™ YM 17 BCYE -

View Our Full Water Sampling Vials Product Offering
TABLE OF CONTENTS Environmental Monitoring 1 Sample Prep and Dilution 8 Dehydrated Culture Media - Criterion™ 12 Prepared Culture Media 14 Chromogenic Media - HardyCHROM™ 18 CompactDry™ 20 Quality Control 24 Membrane Filtration 25 Rapid Tests 26 Lab Supplies/Sample Collection 27 Made in the USA Headquarters Distribution Centers 1430 West McCoy Lane Santa Maria, California Santa Maria, CA 93455 Olympia, Washington 800.266.2222 : phone Salt Lake City, Utah [email protected] Phoenix, Arizona HardyDiagnostics.com Dallas, Texas Springboro, Ohio Hardy Diagnostics has a Quality Lake City, Florida Management System that is certified Albany, New York to ISO 13485 and is a FDA licensed Copyright © 2020 Hardy Diagnostics Raleigh, North Carolina medical device manufacturer. Environmental Monitoring Hardy Diagnostics offers a wide selection of quality products intended to help keep you up to date with regulatory compliance, ensure the efficacy of your cleaning protocol, and properly monitor your environment. 800.266.2222 [email protected] HardyDiagnostics.com 1 Environmental Monitoring Air Sampling TRIO.BAS™ Impact Air Samplers introduced the newest generation of microbial air sampling. These ergonomically designed instruments combine precise air sampling with modern connectivity to help you properly assess the air quality of your laboratory and simplify your process. MONO DUO Each kit includes: Each kit includes: TRIO.BAS™ MONO air sampler, induction TRIO.BAS™ DUO air sampler, battery battery charger and cable, aspirating charger -

BD Industry Catalog
PRODUCT CATALOG INDUSTRIAL MICROBIOLOGY BD Diagnostics Diagnostic Systems Table of Contents Table of Contents 1. Dehydrated Culture Media and Ingredients 5. Stains & Reagents 1.1 Dehydrated Culture Media and Ingredients .................................................................3 5.1 Gram Stains (Kits) ......................................................................................................75 1.1.1 Dehydrated Culture Media ......................................................................................... 3 5.2 Stains and Indicators ..................................................................................................75 5 1.1.2 Additives ...................................................................................................................31 5.3. Reagents and Enzymes ..............................................................................................75 1.2 Media and Ingredients ...............................................................................................34 1 6. Identification and Quality Control Products 1.2.1 Enrichments and Enzymes .........................................................................................34 6.1 BBL™ Crystal™ Identification Systems ..........................................................................79 1.2.2 Meat Peptones and Media ........................................................................................35 6.2 BBL™ Dryslide™ ..........................................................................................................80 -
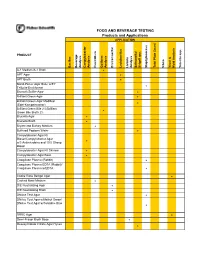
DONE Food and Beverage Testing
FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. A-1 Medium/A-1 Broth ! APT Agar ! APT Broth ! Baird-Parker Agar Base w/EY Tellurite Enrichment ! Bismuth Sulfite Agar ! Brilliant Green Agar ! Brilliant Green Agar Modified (Edel-Kampelmacher) ! Brilliant Green Bile 2%/Brilliant Green Bile Broth 2% ! Brucella Agar ! Brucella Broth ! Bryant and Burkey Medium ! Buffered Peptone Water ! Campylobacter Agar Kit Blaser/Campylobacter Agar w/5 Antimicrobics and 10% Sheep ! Blood Campylobacter Agar Kit Skirrow ! Campylobacter Agar Base ! Coagulase Plasma (Rabbit) ! Coagulase Plasma EDTA (Rabbit)/ Coagulase Plasma w/EDTA ! Cooke Rose Bengal Agar ! Cooked Meat Medium ! D/E Neutralizing Agar ! D/E Neutralizing Broth ! DNAse Test Agar ! DNAse Test Agar w/Methyl Green/ DNAse Test Agar w/Toluidine Blue ! DRBC Agar ! Demi-Fraser Broth Base ! Desoxycholate Citrate Agar Hynes ! FOOD AND BEVERAGE TESTING Products and Applications APPLICATION PRODUCT Bacillus Beverage Analysis Campylobacter Analysis Clostridium Coliform Analysis Environmental Lactobacillus Listeria Analysis Salmonella/ Shigell spp. Staphylococcus Total Plate Count Vibrio Yeast & Mold Analysis Yersinia spp. Differential Reinforced Clostridial Agar ! EC Medium/EC Broth ! EC Medium with MUG/EC Broth w/MUG ! Elliker Broth ! m Endo Agar LES ! m Endo -

Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation Using Lauryl Tryptose Broth (LTB) and EC Medium
United States Office of Water EPA-821-R-14-009 Environmental Protection Agency (4303-T) September 2014 Method 1680: Fecal Coliforms in Sewage Sludge (Biosolids) by Multiple-Tube Fermentation using Lauryl Tryptose Broth (LTB) and EC Medium U.S. Environmental Protection Agency Office of Water (4303T) 1200 Pennsylvania Avenue, NW Washington, DC 20460 Method 1680 Acknowledgments The contributions of the following persons and organizations to this study are gratefully acknowledged: Referee Laboratory • EPA Office of Research and Development, National Risk Management Research Lab: Mark C. Meckes and Karen M. White Volunteer Participant Laboratories • American Interplex: John Overbey and Lizbeth Huggins • BioVir Laboratories: Rick Danielson and Jim Truscott • City of Los Angeles Bureau of Sanitation Environmental Monitoring Division: Farhana Mohamed and Zora Bahariance • County Sanitation Districts of Los Angeles County: Shawn Thompson and Julie Millenbach • Environmental Associates: Susan Boutros and John Chandler • Hampton Roads Sanitation District: Anna Rule and Bob Maunz • King County Environmental Laboratory: Greg Ma and Bobbie Anderson • Hoosier Microbiological Laboratories: Don Hendrickson, Keri Nixon, Katy Bilger, and Lindsey Shelton • Massachusetts Water Resources Authority: Steve Rhode and Mariya Gofshteyn • Milwaukee Metropolitan Sewerage District: Jeff MacDonald and Tim O’Neill • University of Iowa Hygienic Laboratory: Nancy Hall and Cathy Lord • Utah Department of Health: Sanwat Chaudhuri and Devon Cole The following facilities provided -

Mississippi Academy of Sciences
MISSISSIPPI ACADEMY OF SCIENCES EIGHTY-FOURTH ANNUAL MEETING February 20-21, 2020 Mississippi Gulf Coast and Convention Center Biloxi, MS Sponsors (Annual Meeting Sponsor) Mississippi State University- College of Agriculture and Life Sciences College of Forest Resources James Worth Bagley College of Engieering Journal of the Mississippi Academy of Sciences Volume 65 January 2020 Number 1 Contents 3 ACADEMY OFFICERS & DIVISION CHAIRS 2019-2020 Editor 4 GENERAL SCHEDULE Michelle Tucci University of Mississippi Medical Center 7 DIRECTIONS TO CONFERENCE CENTER Editorial Board 8 SUSTAINING MEMBERS 9 LIFE MEMBERS Maria Begonia Jackson State University 10 EXHIBITORS 11 EXECUTIVE DIRECTOR’S COLUMN Ibrahim O. Farah 12 MAS AWARDS AND DODGEN LECTURE Jackson State University 16 SPECIAL PRESENTATIONS Millsaps Undergraduate Student Awards and Mississippi INBRE Robin Rockhold Graduate Student Awards, LSMAMP University of Mississippi Medical Center Ham Benghuzzi 24 OVERVIEW OF DIVISIONAL PROGRAMS University of Mississippi Medical Center Biloxi Convention Center Floor Plans and Notes Program Editor ABSTRACTS Kenneth Butler 36 Agriculture and Plant Science University of Mississippi Medical Center 43 Cellular, Molecular and Developmental Biology 63 Chemistry and Chemical Engineering The Journal of the Mississippi Academy of Sciences (ISSN 0076-9436) is published in 88 Ecology and Evolutionary Biology January (annual meeting abstracts), April, 94 Geology and Geography July, and October, by the Mississippi Acad- emy of Sciences. Members of the Academy 101 Health Sciences receive the journal as part of their regular (nonstudent) membership. Inquiries 123 History and Philosophy of Science regarding subscriptions, availability of back 127 Marine and Atmospheric Sciences issues, and address changes should be addressed to The Mississippi Academy of 130 Mathematics, Computer Science and Statistics Sciences, Post Office Box 55709, Jackson, 136 Physics and Engineering MS 39296-5709, telephone 601-977-0627, or email [email protected]. -

CDC Anaerobe 5% Sheep Blood Agar with Phenylethyl Alcohol (PEA) CDC Anaerobe Laked Sheep Blood Agar with Kanamycin and Vancomycin (KV)
Difco & BBL Manual Manual of Microbiological Culture Media Second Edition Editors Mary Jo Zimbro, B.S., MT (ASCP) David A. Power, Ph.D. Sharon M. Miller, B.S., MT (ASCP) George E. Wilson, MBA, B.S., MT (ASCP) Julie A. Johnson, B.A. BD Diagnostics – Diagnostic Systems 7 Loveton Circle Sparks, MD 21152 Difco Manual Preface.ind 1 3/16/09 3:02:34 PM Table of Contents Contents Preface ...............................................................................................................................................................v About This Manual ...........................................................................................................................................vii History of BD Diagnostics .................................................................................................................................ix Section I: Monographs .......................................................................................................................................1 History of Microbiology and Culture Media ...................................................................................................3 Microorganism Growth Requirements .............................................................................................................4 Functional Types of Culture Media ..................................................................................................................5 Culture Media Ingredients – Agars ...................................................................................................................6 -

The Existing Literature in Connection with the Bacteriology
‘The existing literature in connection with the Bacteriology of Water is so extensive, that we venture to think an urgent necessity has arisen for a work in which this subject is specially discussed.’ Percy Frankland, 1894 Micro-organisms in Water – Their significance, identification and removal Promoter Prof. dr. ir. Nico Boon Department of Biochemical and Microbial Technology, Faculty of Bioscience Engineering, Ghent University, Belgium Co-promoter Dr. Frederik Hammes Department of Environmental Microbiology, Eawag: Swiss Federal Institute of Aquatic Science and Technology, Switzerland Members of the examination committee Dr. Ameet Pinto School of Engineering, University of Glasgow, United Kingdom Ing. Sabine Kreps VITO: Flemish Institute for Technological Research, Belgium Prof. dr. ir. Mieke Uyttendaele Department of Food Safety and Food Quality, Faculty of Bioscience Engineering, Ghent University, Belgium Prof. dr. ir. Arne Verliefde Department of Applied Analytical and Physical Chemistry, Faculty of Bioscience Engineering, Ghent University, Belgium Dean Faculty of Bioscience Engineering Prof. dr. ir. Guido Van Huylenbroeck Rector Ghent University Prof. dr. Anne De Paepe GROWTH AND FLOW CYTOMETRIC MONITORING OF BACTERIA IN DRINKING WATER IR. SAM VAN NEVEL Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Environmental Technology Dutch translation of the title Groei en flowcytometrische monitoring van bacteriën in drinkwater Funding This work was supported by the Research Foundation – Flanders (Fonds voor Wetenschappelijk Onderzoek (FWO) Vlaanderen), project grant no. G.0808.10N. Cover illustration Cover model: Sanne Van Nevel Flow cytometry scheme Network map FARYS © Timternet Infiltration pond St-André IWVA, Oostduinkerke Van Nevel S. (2014) Growth and flow cytometric monitoring of bacteria in drinking water. -
![Foodborne Pathogens Testing Guide [EN]](https://docslib.b-cdn.net/cover/4751/foodborne-pathogens-testing-guide-en-4334751.webp)
Foodborne Pathogens Testing Guide [EN]
Thermo Scientific Food Testing Solutions Solutions for the detection of foodborne pathogens Easily and accurately identify foodborne pathogens using Thermo Scientific culture and rapid biochemical test products. Contents of Foodborne Organisms Salmonella Listeria monocytogenes Escherichia coli and Coliforms Diarrheagenic Escherichia coli Campylobacter Staphylococcus aureus Bacillus cereus Clostridium perfingens Shigella Vibrio Yeast and Mold Products available for the detection, isolation and identification of foodborne pathogens include but are not limited to the organisms listed in this reference. For more information on our complete range of Thermo Scientific microbiology solutions, please contact your local sales representative at 1-800-255-6730, or visit www.thermoscientific.com/remel. Thermo Scientific Food Testing Solutions The Thermo Scientific microbiology portfolio includes an extensive range of products for the isolation, identification and enumeration of foodborne pathogens. These products range from culture media and diagnostic kits to quality control organisms. Our focus on providing quality products, on-time delivery and superior support is matched by our commitment to provide complete solutions that meet your testing needs. The Thermo Scientific Food Testing Solutions guide illustrates how our Oxoid and Remel brand microbiology products fit into the workflow of a food testing laboratory. With the recent publicity regarding food-related outbreaks and illnesses, now is the time to respond with accurate and reliable solutions. Culture Media Utilizing the latest technology in our state-of-the-art, FDA and ISO compliant manufacturing facility and backed by a team of experts dedicated to microbiology, we deliver the high-quality culture media your laboratory can depend on. • Thermo Scientific Oxoid Dry-BagsTM save time in bulk preparation and dispensing of media in food laboratories. -

Lauryl Tryptose Broth
HiMedia Laboratories Technical Data Lauryl Sulphate Broth (Lauryl Tryptose Broth) M080 Lauryl Sulphate Broth (Lauryl Tryptose Broth) is used for the detection of coliforms in water, wastewater, dairy products and other food samples. Composition*** Ingredients Gms / Litre Tryptose 20.000 Lactose 5.000 Sodium chloride 5.000 Dipotassium phosphate 2.750 Monopotassium phosphate 2.750 Sodium lauryl sulphate 0.100 Final pH ( at 25°C) 6.8±0.2 **Formula adjusted, standardized to suit performance parameters Directions Suspend 35.6 grams in 1000 ml distilled water. Heat if necessary to dissolved the medium completely. Distribute into tubes containing inverted Durhams tubes. Sterilize by autoclaving at 15 lbs pressure (121°C) for 15 minutes. For inoculum of 1 ml or less, use single strength medium. For inocula of 10 ml or more, double strength or proportionate medium should be prepared. Principle And Interpretation Coliforms are considered to be members of Enterobacteriaceae , which grow in the presence of bile salts and produce acid and gas from lactose within 48 hours at 37°C (1). These bacteria can also be defined as, members of Enterobacteriaceae capable of growing at 37°C, that normally possess b-galactosidase (2). Lauryl Sulphate Broth is used for the detection of coliforms in water, dairy products and other foods, as recommended by APHA (3, 4, 5). It can also be used for the presumptive detection of coliforms in water, effluent or sewage by the MPN test (3). Lauryl Sulphate Broth was developed by Mallmann and Darby (6). Cowls (7) demonstrated that inclusion of sodium lauryl sulphate makes the medium selective for coliform bacteria. -

Microbiology Research Volume 10 Number 33 7 September, 2016 ISSN 1996-0808
African Journal of Microbiology Research Volume 10 Number 33 7 September, 2016 ISSN 1996-0808 The African Journal of Microbiology Research (AJMR) is published weekly (one volume per year) by Academic Journals. provides rapid publication (weekly) of articles in all areas of Microbiology such as: Environmental Microbiology, Clinical Microbiology, Immunology, Virology, Bacteriology, Phycology, Mycology and Parasitology, Protozoology, Microbial Ecology, Probiotics and Prebiotics, Molecular Microbiology, Biotechnology, Food Microbiology, Industrial Microbiology, Cell Physiology, Environmental Biotechnology, Genetics, Enzymology, Molecular and Cellular Biology, Plant Pathology, Entomology, Biomedical Sciences, Botany and Plant Sciences, Soil and Environmental Sciences, Zoology, Endocrinology, Toxicology. The Journal welcomes the submission of manuscripts that meet the general criteria of significance and scientific excellence. Papers will be published shortly after acceptance. All articles are peer-reviewed. Contact Us Editorial Office: [email protected] Help Desk: [email protected] Website: http://www.academicjournals.org/journal/AJMR Submit manuscript online http://ms.academicjournals.me/ Editors Dr. Thaddeus Ezeji Prof. Stefan Schmidt Applied and Environmental Microbiology Fermentation and Biotechnology Unit School of Biochemistry, Genetics and Microbiology Department of Animal Sciences University of KwaZulu-Natal The Ohio State University Pietermaritzburg, USA. South Africa. Dr. Mamadou Gueye Prof. Fukai Bao MIRCEN/Laboratoire