Composition, Stability, and Structure of a New Member of the Aenigmatite Group, Na2mg4+Xfe Si6+Xo20, Synthesized at 13–14
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
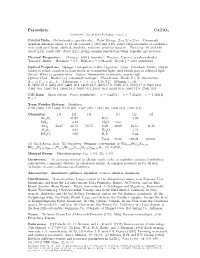
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Petrology of Nepheline Syenite Pegmatites in the Oslo Rift, Norway: Zr and Ti Mineral Assemblages in Miaskitic and Agpaitic Pegmatites in the Larvik Plutonic Complex
MINERALOGIA, 44, No 3-4: 61-98, (2013) DOI: 10.2478/mipo-2013-0007 www.Mineralogia.pl MINERALOGICAL SOCIETY OF POLAND POLSKIE TOWARZYSTWO MINERALOGICZNE __________________________________________________________________________________________________________________________ Original paper Petrology of nepheline syenite pegmatites in the Oslo Rift, Norway: Zr and Ti mineral assemblages in miaskitic and agpaitic pegmatites in the Larvik Plutonic Complex Tom ANDERSEN1*, Muriel ERAMBERT1, Alf Olav LARSEN2, Rune S. SELBEKK3 1 Department of Geosciences, University of Oslo, PO Box 1047 Blindern, N-0316 Oslo Norway; e-mail: [email protected] 2 Statoil ASA, Hydroveien 67, N-3908 Porsgrunn, Norway 3 Natural History Museum, University of Oslo, Sars gate 1, N-0562 Oslo, Norway * Corresponding author Received: December, 2010 Received in revised form: May 15, 2012 Accepted: June 1, 2012 Available online: November 5, 2012 Abstract. Agpaitic nepheline syenites have complex, Na-Ca-Zr-Ti minerals as the main hosts for zirconium and titanium, rather than zircon and titanite, which are characteristic for miaskitic rocks. The transition from a miaskitic to an agpaitic crystallization regime in silica-undersaturated magma has traditionally been related to increasing peralkalinity of the magma, but halogen and water contents are also important parameters. The Larvik Plutonic Complex (LPC) in the Permian Oslo Rift, Norway consists of intrusions of hypersolvus monzonite (larvikite), nepheline monzonite (lardalite) and nepheline syenite. Pegmatites ranging in composition from miaskitic syenite with or without nepheline to mildly agpaitic nepheline syenite are the latest products of magmatic differentiation in the complex. The pegmatites can be grouped in (at least) four distinct suites from their magmatic Ti and Zr silicate mineral assemblages. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -

Paleoproterozoic Late-Orogenic and Anorogenic Alkaline Granitic Magmatism from Northeast Brazil
Precambrian Research 104 (2000) 47–75 www.elsevier.com/locate/precamres Paleoproterozoic late-orogenic and anorogenic alkaline granitic magmatism from northeast Brazil J. Pla´ Cid a,*, M.F. Bitencourt a, L.V.S. Nardi a, H. Conceic¸a˜o b, B. Bonin c, J.M. Lafon d a Curso de Po´s-Graduac¸a˜o em in Geocieˆncias, Campus de Agronomia, Instituto de Geociencias, Uni6ersity Federal do Rio Grande do Sul (UFRGS), A6. Bento Gonc¸al6es, 9500, CEP-91509-900 Porto Alegre, RS, Brazil b CPGG-PPPG/UFBA, Rua Caetano Moura, 123, Instituto de Geocieˆncias, UFBA, CEP-40210-350, Sal6ador, BA, Brazil c Departement des Sciences de la Terre, Laboratoire de Pe´trographie et Volcanologie, Uni6ersite´ Paris, Sud. Centre d’Orsay, Bat. 504, F-91504 Paris, France d Centro de Geocieˆncias, Laborato´rio de Geologia Isoto´pica, Uni6ersidade Federal do Para´ (UFPA), Para´, Brazil Received 21 January 1999; accepted 4 May 2000 Abstract During the Transamazonian cycle (2.090.2 Ga), two silica-saturated alkaline granite suites were intruded along the border of the Sa˜o Francisco craton, northeastern Brazil. The Couro de Onc¸a intrusive suite (COIS) is late tectonic relative to major deformational events, and the Serra do Meio suite (SMS) postdates this event, being interpreted as anorogenic type, deformed during the Brasiliano cycle (0.65–0.52 Ga). The COIS encompasses alkali-feldspar granites, syenogranites, monzogranites, with dykes of similar composition and age (2.15795 Ma). They are metaluminous rocks of alkaline affinity, whose evolution was probably controlled by mineral fractionation processes involving mainly plagioclase, amphibole, and Fe–Ti oxide. -

DISCOVERY of NEW MINERAL ADDIBISCHOFFITE, Ca2al6al6o20, in a Ca-Al-RICH REFRACTORY INCLUSION from the ACFER 214 CH3 METEORITE. Chi Ma1,*, Alexander N
79th Annual Meeting of the Meteoritical Society (2016) 6016.pdf DISCOVERY OF NEW MINERAL ADDIBISCHOFFITE, Ca2Al6Al6O20, IN A Ca-Al-RICH REFRACTORY INCLUSION FROM THE ACFER 214 CH3 METEORITE. Chi Ma1,*, Alexander N. Krot2, Kazuhide Nagashima2. 1Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125, USA; 2Hawai‛i Institute of Geophysics and Planetology, University of Hawai‛i at Mānoa, Honolulu, HI 96822, USA; *Email: [email protected]. Introduction: During a mineralogy investigation of the Acfer 214 CH3 carbonaceous chondrite, a new calcium aluminate mineral, named “addibischoffite”, Ca2Al6Al6O20 with the P-1 aenigmatite structure, was identified in a Ca-Al-rich inclusion (CAI). Field-emission scanning electron microscope, electron back-scatter diffraction, electron microprobe and ion microprobe were used to characterize its chemical and oxygen-isotope compositions, structure, and associated phases. Synthetic CaAl6O10 was reported but not fully characterized [e.g., 1]. Presented here is its first natural occurrence as a new refractory mineral in a primitive meteorite. The mineral has been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA 2015-006). The name is in honor of Addi Bischoff, a cosmochemist at Münster University, Germany, for his many contributions to research on CAIs in carbonaceous chondrites, including CH chondrites. Occurrence, Chemistry, Oxygen Isotopes and Crystallography: Addibischoffite occurs as one irregular crys- tal, 9 μm × 3.5 μm in size, with hibonite, perovskite, kushiroite, spinel, melilite, anorthite, Ni-bearing iron in the mantle-center area of this CAI, surrounded by an igneous Ti-poor Al-diopside rim intergrown with small grains of low-Ca pyroxene (Fig. -

Carbonatites of the World, Explored Deposits of Nb and REE—Database and Grade and Tonnage Models
Carbonatites of the World, Explored Deposits of Nb and REE—Database and Grade and Tonnage Models By Vladimir I. Berger, Donald A. Singer, and Greta J. Orris Open-File Report 2009-1139 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2009 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod/ Telephone: 1-888-ASK-USGS For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web: http://www.usgs.gov/ Telephone: 1-888-ASK-USGS Suggested citation: Berger, V.I., Singer, D.A., and Orris, G.J., 2009, Carbonatites of the world, explored deposits of Nb and REE— database and grade and tonnage models: U.S. Geological Survey Open-File Report 2009-1139, 17 p. and database [http://pubs.usgs.gov/of/2009/1139/]. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. ii Contents Introduction 1 Rules Used 2 Data Fields 2 Preliminary analysis: —Grade and Tonnage Models 13 Acknowledgments 16 References 16 Figures Figure 1. Location of explored Nb– and REE–carbonatite deposits included in the database and grade and tonnage models 4 Figure 2. Cumulative frequency of ore tonnages of Nb– and REE–carbonatite deposits 14 Figure 3 Cumulative frequency of Nb2O5 grades of Nb– and REE–carbonatite deposits 15 Figure 4 Cumulative frequency of RE2O3 grades of Nb– and REE–carbonatite deposits 15 Figure 4 Cumulative frequency of P2O5 grades of Nb– and REE–carbonatite deposits 16 Tables Table 1. -

Natural Titanite Reference Materials for in Situ U‐Pb and Sm‐Nd Isotopic Measurements by LA‐(MC)‐ICP‐MS
Vol. 43 — N° 3 09 p.355– 384 19 Natural Titanite Reference Materials for In Situ U-Pb and Sm-Nd Isotopic Measurements by LA-(MC)-ICP-MS Qian Ma (1, 2),NoreenJ.Evans (3), Xiao-Xiao Ling (2, 4),Jin-HuiYang (2, 4),Fu-YuanWu (2, 4), Zhi-Dan Zhao (1)* and Yue-Heng Yang (2, 4)* (1) State Key Laboratory of Geological Processes and Mineral Resources, School of Earth Science and Resources, China University of Geosciences, Beijing 100083, China (2) State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China (3) School of Earth and Planetary Science, John de Laeter Centre, Curtin University, Perth, WA, Australia (4) Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China * Corresponding authors. e-mails: [email protected] and [email protected] Titanite is a common accessory mineral that preferentially incorporates considerable amounts of U and light rare earth elements in its structure, making it a versatile mineral for in situ U-Pb dating and Sm-Nd isotopic measurement. Here, we present in situ U-Pb ages and Sm-Nd isotope measurement results for four well-known titanite reference materials (Khan, BLR-1, OLT1 and MKED1) and eight titanite crystals that could be considered potential reference material candidates (Ontario, YQ-82, T3, T4, TLS-36, NW-IOA, Pakistan and C253), with ages ranging from ~ 20 Ma to ~ 1840 Ma. Results indicate that BLR-1, OLT1, Ontario, MKED1 and T3 titanite have relatively homogeneous Sm-Nd isotopes and low common Pb and thus can serve as primary reference materials for U-Pb and Sm-Nd microanalysis.