The Propertiesof Anatase Pseudomorphs After
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

19660017397.Pdf
.. & METEORITIC RUTILE Peter R. Buseck Departments of Geology and Chemistry Arizona State University Tempe, Arizona Klaus Keil Space Sciences Division National Aeronautics and Space Administration Ames Research Center Mof fett Field, California r ABSTRACT Rutile has not been widely recognized as a meteoritic constituent. show, Recent microscopic and electron microprobe studies however, that Ti02 . is a reasonably widespread phase, albeit in minor amounts. X-ray diffraction studies confirm the Ti02 to be rutile. It was observed in the following meteorites - Allegan, Bondoc, Estherville, Farmington, and Vaca Muerta, The rutile is associated primarily with ilmenite and chromite, in some cases as exsolution lamellae. Accepted for publication by American Mineralogist . Rutile, as a meteoritic phase, is not widely known. In their sunanary . of meteorite mineralogy neither Mason (1962) nor Ramdohr (1963) report rutile as a mineral occurring in meteorites, although Ramdohr did describe a similar phase from the Faxmington meteorite in his list of "unidentified minerals," He suggested (correctly) that his "mineral D" dght be rutile. He also ob- served it in several mesosiderites. The mineral was recently mentioned to occur in Vaca Huerta (Fleischer, et al., 1965) and in Odessa (El Goresy, 1965). We have found rutile in the meteorites Allegan, Bondoc, Estherville, Farming- ton, and Vaca Muerta; although nowhere an abundant phase, it appears to be rather widespread. Of the several meteorites in which it was observed, rutile is the most abundant in the Farmington L-group chondrite. There it occurs in fine lamellae in ilmenite. The ilmenite is only sparsely distributed within the . meteorite although wherever it does occur it is in moderately large clusters - up to 0.5 mn in diameter - and it then is usually associated with chromite as well as rutile (Buseck, et al., 1965), Optically, the rutile has a faintly bluish tinge when viewed in reflected, plane-polarized light with immersion objectives. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine
nanomaterials Review Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine Daniel Ziental 1 , Beata Czarczynska-Goslinska 2, Dariusz T. Mlynarczyk 3 , Arleta Glowacka-Sobotta 4, Beata Stanisz 5, Tomasz Goslinski 3,* and Lukasz Sobotta 1,* 1 Department of Inorganic and Analytical Chemistry, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 2 Department of Pharmaceutical Technology, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 3 Department of Chemical Technology of Drugs, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] 4 Department and Clinic of Maxillofacial Orthopedics and Orthodontics, Poznan University of Medical Sciences, Bukowska 70, 60-812 Poznan, Poland; [email protected] 5 Department of Pharmaceutical Chemistry, Poznan University of Medical Sciences, Grunwaldzka 6, 60-780 Poznan, Poland; [email protected] * Correspondence: [email protected] (T.G.); [email protected] (L.S.) Received: 4 January 2020; Accepted: 19 February 2020; Published: 23 February 2020 Abstract: Metallic and metal oxide nanoparticles (NPs), including titanium dioxide NPs, among polymeric NPs, liposomes, micelles, quantum dots, dendrimers, or fullerenes, are becoming more and more important due to their potential use in novel medical therapies. Titanium dioxide (titanium(IV) oxide, titania, TiO2) is an inorganic compound that owes its recent rise in scientific interest to photoactivity. After the illumination in aqueous media with UV light, TiO2 produces an array of reactive oxygen species (ROS). The capability to produce ROS and thus induce cell death has found application in the photodynamic therapy (PDT) for the treatment of a wide range of maladies, from psoriasis to cancer. -

Electronic Structure and Optical Properties of Prominent Phases of Tio2: First-Principles Study
Pramana – J. Phys. (2017) 89:5 © Indian Academy of Sciences DOI 10.1007/s12043-017-1400-5 Electronic structure and optical properties of prominent phases of TiO2: First-principles study SANTOSH SINGH and MADHVENDRA NATH TRIPATHI∗ Department of Pure and Applied Physics, Guru Ghasidas Vishwavidyalaya (Central University), Koni, Bilaspur 495 009, India ∗Corresponding author. E-mail: [email protected] Published online 19 June 2017 Abstract. First-principles study based on density functional theory (DFT) of two prominent phases, the rutile and the anatase phases, of titanium dioxide (TiO2) are reported within the generalized gradient approximation (GGA). Our calculated band structure shows that there is a significant presence of O-2p and Ti-3d hybridization in the valence bands. These bands are well separated from the conduction bands by a direct band gap value of 1.73 eV in the rutile phase and an indirect band gap value of 2.03 eV in the anatase phase, from to X. Our calculations reproduced the peaks in the conduction and valence band, are in good agreement with experimental observations. Our structural optimization for the rutile and anatase phase led to lattice parameter values of 4.62 Å and 2.99 Å rutile and 3.80 Å and 9.55 Å for anatase for a and c. The static dielectric values 7.0 and 5.1 for the rutile and anatase phases respectively are in excellent agreement with experimental results. Our calculation of optical properties reveals that maximum value of the transmittance in anatase phase of TiO2 may be achieved by considering the anisotropic behaviour of the optical spectra in the optical region for transparent conducting application. -

Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (Tio2) and Ti Metal
Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (TiO2) and Ti metal Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Objective To produce ultra pure high grade synthetic rutile (TiO2) from ilmenite ore and Ti metal from anatase or rutile Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Outline 1. Introduction 2. Why titaniferous minerals? 3. Processing Methods for synthetic rutile (TiO2) (i) Alkali Roasting (ii) Reduction followed by leaching 5. Results and discussion 6. Commercialisation 7. Conclusion 8. Bradford Metallurgy on Ti metal powder production 9. Future Plans Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Introduction • Titanium always exist as bonded to other elements in nature. • It is the ninth-most abundant element in the Earth. • It is widely distributed and occurs primarily in the minerals such as anatase, brookite, ilmenite, perovskite, rutile and titanite (sphene). • Among these minerals, only rutile and ilmenite have economic importance Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Ilmenite deposit in Chavara, Kerala, India Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Applications of TiO2 • White powder pigment - brightness and very high refractive index - Sunscreens use TiO2 - high refractive index - protect the skin from UV light. • TiO2 – photocatalysts - electrolytic splitting of water into hydrogen and oxygen, - produce electricity in nanoparticle form -light-emitting diodes, etc. -

Combustion Synthesis, Sintering, Characterization, and Properties
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 9639016, 9 pages https://doi.org/10.1155/2019/9639016 Research Article Calcium Titanate from Food Waste: Combustion Synthesis, Sintering, Characterization, and Properties Siriluk Cherdchom,1 Thitiwat Rattanaphan,1 and Tawat Chanadee 1,2 1Department of Materials Science and Technology, Faculty of Science, Prince of Songkla University (PSU), Hat Yai, Songkhla 90110, &ailand 2Ceramic and Composite Materials Engineering Research Group (CMERG), Center of Excellence in Materials Engineering (CEME), Prince of Songkla University (PSU), Hat Yai, Songkhla 90110, &ailand Correspondence should be addressed to Tawat Chanadee; [email protected] Received 31 August 2018; Revised 20 December 2018; Accepted 3 January 2019; Published 12 February 2019 Academic Editor: Andres Sotelo Copyright © 2019 Siriluk Cherdchom et al. )is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3) was combustion synthesized from a calcium source of waste duck eggshell, anatase titanium dioxide (A-TiO2), and magnesium (Mg). )e eggshell and A-TiO2 were milled for 30 min in either a high-energy planetary mill or a conventional ball mill. )ese powders were then separately mixed with Mg in a ball mill. After synthesis, the combustion products were leached and then sintered to produce CaTiO3 ceramic. Analytical characterization of the as-leached combustion products revealed that the product of the combustion synthesis of duck eggshell + A-TiO2 that had been high-energy-milled for 30 min before synthesis comprised a single perovskite phase of CaTiO3. -

CRYSTAL SRUKTUR of CALCIUM TITANATE (Catio3) PHOSPHOR DOPED with PRASEODYMIUM and ALUMINIUM IONS
CRYSTAL SRUKTUR OF CALCIUM TITANATE (CaTiO3) PHOSPHOR DOPED WITH PRASEODYMIUM AND ALUMINIUM IONS STRUKTUR KRISTAL FOSFOR KALSIUM TITANIA DIDOPKAN DENGAN ION PRASEODYMIUM DAN ALUMINIUM IONS Siti Aishah Ahmad Fuzi1* and Rosli Hussin2 1 Material Technology Group, Industrial Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia. 2 Department of Physics, Faculty of Science, Universiti Teknologi Malaysia, 81310 Skudai, Johor 1*[email protected], [email protected] Abstract The past three decades have witnessed rapid growth in research and development of luminescence phenomenon because of their diversity in applications. In this paper, Calcium Titanate (CaTiO3) was studied to find a new host material with desirable structural properties for luminescence-based applications. Solid state reactions o 3+ methods were used to synthesis CaTiO3 at 1000 C for 6 hours. Crystal structure of CaTiO3 co-doped with Pr 3+ and Al were investigated using X-Ray Diffraction (XRD) method. Optimum percentage to synthesis CaTiO3 was 3+ obtained at 40 mol%CaO-60 mol%TiO2 with a single doping of 1 mol%Pr . However, a crystal structure of 4 mol% of Al3+ co-doped with Pr3+ was determined as an optimum parameter which suitable for display imaging. Keywords: calcium titanate, anatase, rutile Abstrak Semenjak tiga dekad yang lalu telah menunjukkan peningkatan yang ketara bagi kajian dan pembangunan dalam bidang fotolumiscen. Peningkatan ini berkembang dengan meluas disebabkan oleh kebolehannya untuk diaplikasikan dalam pelbagai kegunaan harian. Dalam manuskrip ini, kalsium titania (CaTiO3) telah dikaji untuk mencari bahan perumah dengan sifat struktur yang bersesuaian bagi aplikasi luminescen. Tindak balas keadaan o pepejal telah digunakan bagi mensintesis CaTiO3 pada suhu 1000 C selama 6 jam. -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
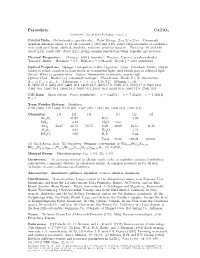
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr.