Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

19660017397.Pdf
.. & METEORITIC RUTILE Peter R. Buseck Departments of Geology and Chemistry Arizona State University Tempe, Arizona Klaus Keil Space Sciences Division National Aeronautics and Space Administration Ames Research Center Mof fett Field, California r ABSTRACT Rutile has not been widely recognized as a meteoritic constituent. show, Recent microscopic and electron microprobe studies however, that Ti02 . is a reasonably widespread phase, albeit in minor amounts. X-ray diffraction studies confirm the Ti02 to be rutile. It was observed in the following meteorites - Allegan, Bondoc, Estherville, Farmington, and Vaca Muerta, The rutile is associated primarily with ilmenite and chromite, in some cases as exsolution lamellae. Accepted for publication by American Mineralogist . Rutile, as a meteoritic phase, is not widely known. In their sunanary . of meteorite mineralogy neither Mason (1962) nor Ramdohr (1963) report rutile as a mineral occurring in meteorites, although Ramdohr did describe a similar phase from the Faxmington meteorite in his list of "unidentified minerals," He suggested (correctly) that his "mineral D" dght be rutile. He also ob- served it in several mesosiderites. The mineral was recently mentioned to occur in Vaca Huerta (Fleischer, et al., 1965) and in Odessa (El Goresy, 1965). We have found rutile in the meteorites Allegan, Bondoc, Estherville, Farming- ton, and Vaca Muerta; although nowhere an abundant phase, it appears to be rather widespread. Of the several meteorites in which it was observed, rutile is the most abundant in the Farmington L-group chondrite. There it occurs in fine lamellae in ilmenite. The ilmenite is only sparsely distributed within the . meteorite although wherever it does occur it is in moderately large clusters - up to 0.5 mn in diameter - and it then is usually associated with chromite as well as rutile (Buseck, et al., 1965), Optically, the rutile has a faintly bluish tinge when viewed in reflected, plane-polarized light with immersion objectives. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (Tio2) and Ti Metal
Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (TiO2) and Ti metal Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Objective To produce ultra pure high grade synthetic rutile (TiO2) from ilmenite ore and Ti metal from anatase or rutile Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Outline 1. Introduction 2. Why titaniferous minerals? 3. Processing Methods for synthetic rutile (TiO2) (i) Alkali Roasting (ii) Reduction followed by leaching 5. Results and discussion 6. Commercialisation 7. Conclusion 8. Bradford Metallurgy on Ti metal powder production 9. Future Plans Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Introduction • Titanium always exist as bonded to other elements in nature. • It is the ninth-most abundant element in the Earth. • It is widely distributed and occurs primarily in the minerals such as anatase, brookite, ilmenite, perovskite, rutile and titanite (sphene). • Among these minerals, only rutile and ilmenite have economic importance Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Ilmenite deposit in Chavara, Kerala, India Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Applications of TiO2 • White powder pigment - brightness and very high refractive index - Sunscreens use TiO2 - high refractive index - protect the skin from UV light. • TiO2 – photocatalysts - electrolytic splitting of water into hydrogen and oxygen, - produce electricity in nanoparticle form -light-emitting diodes, etc. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

74275 Oriented Ilmenite Basalt 1493 Grams
74275 Oriented Ilmenite Basalt 1493 grams 74275 Figure 1: Location of basalt sample 74275 at Shorty Crater - also see 74220. (falce color) AS17-137-20990 Introduction 1981). This sample has proven useful to studies that 74275 is a fine-grained, high-Ti mare basalt with require known lunar orientation with extended significant armalcolite content (Hodges and Kushiro exposure history to the extra-lunar environment 1974; Neal and Taylor 1993). It contains vesicles, vugs (micrometeorites, cosmic rays, solar irradiation). The and unusual olivine megacrysts (Fo82) (Meyer and sample is relatively flat 17 by 12 cm and 4 cm thick. Wilshire 1974). The top (T1) surface is somewhat rounded and has many micrometeorite pits (figure 4), while the bottom 74275 was collected from the rim of Shorty Crater (B1) surface is flat and angular and without any (figure 1) and was photographed both on the lunar evidence of exposure to the micrometeorites (figure surface and in the laboratory (with similar lighting) to 5). Fink et al. (1998) have carefully considered the document the exact lunar orientation. (Wolfe et al. exact orientation (30 deg tilt), shielding (nearby Lunar Sample Compendium C Meyer 2011 boulder) and detailed exposure history (complex) of 74275 74275. Di Hd This basalt has been determined to be very old (> 3.8 b.y.). Based on trace element analysis it is a type C Apollo 17 basalt. Petrography Perhaps the best petrographic description of 74275 is En Fs given by Hodges and Kushiro (1974): “Rock 74275 is a fine-grained ilmenite basalt with microphenocrysts Fo Fa of olivine (Fo80-71), titanaugite (up to 6.8 wt. -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
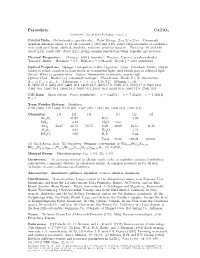
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -
Ceramic Pigments Based on Armalcolite Doped with Vanadium by Mod Methods
CASTELLÓN (ESPAÑA) CERAMIC PIGMENTS BASED ON ARMALCOLITE DOPED WITH VANADIUM BY MOD METHODS C. Gargori, S. Cerro, M. Llusar, G. Monrós Dept. of Inorganic and Organic Chemistry, Universidad Jaume I, Castellón.Spain 1. INTRODUCTION Armalcolite is the structure (orthorhombic, point group 2/m2/m2/m and space group Bbmm) of a mineral detected for the first time in rocks brought from the first lunar exploration in 1969; the name comes from the first letters of the astronauts of this first moon expedition (1): ARMstrong, Aldrin and COLlins. Its stoichiometry and oxidation state vary between the extremes (Mg0,5Fe0,5)Ti2O5 (detected in lunar rocks) and ferroarmalcoli- te (armalcolite, ferrian) (MgFe)(FeTi3)O10 with Fe(II) in the former case and Fe(III) in the latter. Synthetically, it is obtained by calcination at high temperature followed by drastic quenching. If it does not metastabilise by quenching, it breaks down into Mg-ilmenite (Mg-FeTiO3) and rutile. This study examined the possible use of armalcolite stabilised by doping with vanadium (Mg0,5Fe0,5)(VxTi2-x)5O5 as a ceramic pigment. www.qualicer.org 1 CASTELLÓN (ESPAÑA) 2. EXPERIMENTAL AND RESULTS Mg0.5Fe0.5(VxTi2-x)2O5 samples were prepared by the CE route (Ceramic from Mg2(OH)2CO3, iron oxide (III), anatase and NH4VO3), CO (ammoniacal coprecipitation of a solution with NH4VO3 dissolved in nitric acid, Mg2(OH)2CO3, Fe(NO3)3.9H2O and titanium isopropoxide stabilised with acetylacetone) and by the MOD route (metal organic decomposition with additions of a polycarboxylic acid in an oxalic/citric molar ratio: sum of cations = 0 0.25 1 1.5 and 2) (2). -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage.