Metal Oxide Compact Electron Transport Layer Modification For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

19660017397.Pdf
.. & METEORITIC RUTILE Peter R. Buseck Departments of Geology and Chemistry Arizona State University Tempe, Arizona Klaus Keil Space Sciences Division National Aeronautics and Space Administration Ames Research Center Mof fett Field, California r ABSTRACT Rutile has not been widely recognized as a meteoritic constituent. show, Recent microscopic and electron microprobe studies however, that Ti02 . is a reasonably widespread phase, albeit in minor amounts. X-ray diffraction studies confirm the Ti02 to be rutile. It was observed in the following meteorites - Allegan, Bondoc, Estherville, Farmington, and Vaca Muerta, The rutile is associated primarily with ilmenite and chromite, in some cases as exsolution lamellae. Accepted for publication by American Mineralogist . Rutile, as a meteoritic phase, is not widely known. In their sunanary . of meteorite mineralogy neither Mason (1962) nor Ramdohr (1963) report rutile as a mineral occurring in meteorites, although Ramdohr did describe a similar phase from the Faxmington meteorite in his list of "unidentified minerals," He suggested (correctly) that his "mineral D" dght be rutile. He also ob- served it in several mesosiderites. The mineral was recently mentioned to occur in Vaca Huerta (Fleischer, et al., 1965) and in Odessa (El Goresy, 1965). We have found rutile in the meteorites Allegan, Bondoc, Estherville, Farming- ton, and Vaca Muerta; although nowhere an abundant phase, it appears to be rather widespread. Of the several meteorites in which it was observed, rutile is the most abundant in the Farmington L-group chondrite. There it occurs in fine lamellae in ilmenite. The ilmenite is only sparsely distributed within the . meteorite although wherever it does occur it is in moderately large clusters - up to 0.5 mn in diameter - and it then is usually associated with chromite as well as rutile (Buseck, et al., 1965), Optically, the rutile has a faintly bluish tinge when viewed in reflected, plane-polarized light with immersion objectives. -

Washington State Minerals Checklist
Division of Geology and Earth Resources MS 47007; Olympia, WA 98504-7007 Washington State 360-902-1450; 360-902-1785 fax E-mail: [email protected] Website: http://www.dnr.wa.gov/geology Minerals Checklist Note: Mineral names in parentheses are the preferred species names. Compiled by Raymond Lasmanis o Acanthite o Arsenopalladinite o Bustamite o Clinohumite o Enstatite o Harmotome o Actinolite o Arsenopyrite o Bytownite o Clinoptilolite o Epidesmine (Stilbite) o Hastingsite o Adularia o Arsenosulvanite (Plagioclase) o Clinozoisite o Epidote o Hausmannite (Orthoclase) o Arsenpolybasite o Cairngorm (Quartz) o Cobaltite o Epistilbite o Hedenbergite o Aegirine o Astrophyllite o Calamine o Cochromite o Epsomite o Hedleyite o Aenigmatite o Atacamite (Hemimorphite) o Coffinite o Erionite o Hematite o Aeschynite o Atokite o Calaverite o Columbite o Erythrite o Hemimorphite o Agardite-Y o Augite o Calciohilairite (Ferrocolumbite) o Euchroite o Hercynite o Agate (Quartz) o Aurostibite o Calcite, see also o Conichalcite o Euxenite o Hessite o Aguilarite o Austinite Manganocalcite o Connellite o Euxenite-Y o Heulandite o Aktashite o Onyx o Copiapite o o Autunite o Fairchildite Hexahydrite o Alabandite o Caledonite o Copper o o Awaruite o Famatinite Hibschite o Albite o Cancrinite o Copper-zinc o o Axinite group o Fayalite Hillebrandite o Algodonite o Carnelian (Quartz) o Coquandite o o Azurite o Feldspar group Hisingerite o Allanite o Cassiterite o Cordierite o o Barite o Ferberite Hongshiite o Allanite-Ce o Catapleiite o Corrensite o o Bastnäsite -

Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (Tio2) and Ti Metal
Mineral to Metal: Processing of Titaniferous Ore to Synthetic Rutile (TiO2) and Ti metal Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Objective To produce ultra pure high grade synthetic rutile (TiO2) from ilmenite ore and Ti metal from anatase or rutile Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Outline 1. Introduction 2. Why titaniferous minerals? 3. Processing Methods for synthetic rutile (TiO2) (i) Alkali Roasting (ii) Reduction followed by leaching 5. Results and discussion 6. Commercialisation 7. Conclusion 8. Bradford Metallurgy on Ti metal powder production 9. Future Plans Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Introduction • Titanium always exist as bonded to other elements in nature. • It is the ninth-most abundant element in the Earth. • It is widely distributed and occurs primarily in the minerals such as anatase, brookite, ilmenite, perovskite, rutile and titanite (sphene). • Among these minerals, only rutile and ilmenite have economic importance Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Ilmenite deposit in Chavara, Kerala, India Dr Jeya Ephraim, Mineral to Metal May 11 – 13, 2015 * Hilton Birmingham Metropole Hotel * Birmingham, United Kingdom Applications of TiO2 • White powder pigment - brightness and very high refractive index - Sunscreens use TiO2 - high refractive index - protect the skin from UV light. • TiO2 – photocatalysts - electrolytic splitting of water into hydrogen and oxygen, - produce electricity in nanoparticle form -light-emitting diodes, etc. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
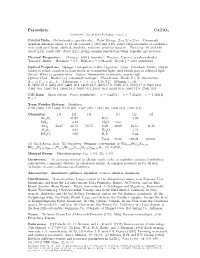
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

Phase Relations in the Systems Titania and Titania--Boric Oxide
This dissertation has been 65—9337 microfilmed exactly as received BEARD, William Clarence, 1934- PHASE RELATIONS IN THE SYSTEMS TITANIA AND TITANIA— BORIC OXIDE. The Ohio State University, Ph.D., 1965 M in eralogy University Microfilms, Inc., Ann Arbor, Michigan PHASE RELATIONS IN THE SYSTEMS TITANIA AND TITANIA—BORIC OXIDE DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University by William Clarence Beard, B. S. The Ohio State University 1965 Approved by n Adviser Department of Mineralogy ACKNOWLEDGMENTS The author wishes to thank the people who have contributed to the preparation of this dissertation. First, to his adviser, Dr. Wilfrid Raymond Foster, for advice and suggestion of the problem; and second to other members of the faculty of the Department of Mineralogy, Drs. Henry Edward Wenden, Ernest George Ehlers, and Rodney Tampa Tettenhorst, for discussions of the problem and the reading of this dissertation, the author extends his thanks. Thanks also go to his colleague, William Charles Butterman, for assistance in the construction and main tenance of many pieces of experimental apparatus. Acknowledgment is made for financial support received under contract No. AF 33(616)-6509 (twenty-four months), spon sored by Aeronautical Research Division, Wright-Patterson Air Force Base, Ohio, as well as for a Mershon National Graduate Fellowship awarded (1962-63) by the Mershon Committee on Education in National Security; and a William J. McCaughey Fellowship (1963-64). The author is indebted to his wife, Ursula, for her constant help and encouragement. ii VITA. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy. -

Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia)
geosciences Article Ti-Nb Mineralization of Late Carbonatites and Role of Fluids in Its Formation: Petyayan-Vara Rare-Earth Carbonatites (Vuoriyarvi Massif, Russia) Evgeniy Kozlov 1,* ID , Ekaterina Fomina 1, Mikhail Sidorov 1 and Vladimir Shilovskikh 2 ID 1 Geological Institute, Kola Science Centre, Russian Academy of Sciences, 14, Fersmana Street, 184209 Apatity, Russia; [email protected] (E.F.); [email protected] (M.S.) 2 Resource center for Geo-Environmental Research and Modeling (GEOMODEL), St. Petersburg State University, 1, Ulyanovskaya Street, 198504 Saint Petersburg, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-953-758-7632 Received: 6 July 2018; Accepted: 25 July 2018; Published: 28 July 2018 Abstract: This article is devoted to the geology of titanium-rich varieties of the Petyayan-Vara rare-earth dolomitic carbonatites in Vuoriyarvi, Northwest Russia. Analogues of these varieties are present in many carbonatite complexes. The aim of this study was to investigate the behavior of high field strength elements during the late stages of carbonatite formation. We conducted a multilateral study of titanium- and niobium-bearing minerals, including a petrographic study, Raman spectroscopy, microprobe determination of chemical composition, and electron backscatter diffraction. Three TiO2-polymorphs (anatase, brookite and rutile) and three pyrochlore group members (hydroxycalcio-, fluorcalcio-, and kenoplumbopyrochlore) were found to coexist in the studied rocks. The formation of these minerals occurred in several stages. First, Nb-poor Ti-oxides were formed in the fluid-permeable zones. The overprinting of this assemblage by residual fluids led to the generation of Nb-rich brookite (the main niobium concentrator in the Petyayan-Vara) and minerals of the pyrochlore group. -

Polymorphism in Bi-Based Perovskite Oxides: a First-Principles Study
Polymorphism in Bi-based Perovskite Oxides: a First-principles Study Oswaldo Diéguez Department of Materials Science and Engineering, Tel Aviv University, Email: [email protected] Web: http://www.eng.tau.ac.il/~dieguez Abstract Under normal conditions, bulk crystals of BiScO3, BiCrO3, BiMnO3, BiFeO3, and BiCoO3 present three very different variations of the perovskite structure: an antipolar phase, a rhombohedral phase with a large polarization along the space diagonal of the pseudocubic unit cell, and a supertetragonal phase with even larger polarization. With the aim of understanding the causes for this variety, we have used a genetic algorithm to search for minima in the surface energy of these materials. Our results show that the number of these minima is very large when compared to that of typical ferroelectric perovskites like BaTiO3 and PbTiO3, and that a fine energy balance between them results in the large structural differences seen. As byproducts of our search we have identified charge-ordering structures with low energy in BiMnO3, and several phases with energies that are similar to that of the ground state of BiCrO3. We have also found that a inverse supertetragonal phase exists in bulk, likely to be favored in films epitaxially grown at large values of tensile misfit strain. Short Biography Dr. Oswaldo Dieguez received his PhD from University of Santiago de Compostela, Spain. After postdoctoral stays at Cambridge, Rutgers, and MIT he joined the Institute of Materials Science of Barcelona as a staff researcher. He moved to the Department of Materials Science and Engineering of Tel Aviv University in 2013, where he is a senior lecturer (assistant professor).