High Efficiency Perovskite Quantum Dot Solar Cells with Charge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
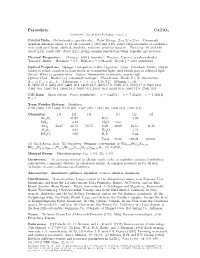
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy. -

Aurivillius Halide Perovskite: a New Family of Two-Dimensional Materials for Optoelectronic Applications
Aurivillius halide perovskite: a new family of two-dimensional materials for optoelectronic applications Shuai Zhaoa,*, Chunfeng Lanb, Huanhuan Lia, Chu Zhangc, Tingli Mac,d aChongqing Key Laboratory of Green Energy Materials Technology and Systems, School of Science, Chongqing University of Technology, Chongqing 400054, P.R. China bSchool of Automotive and Transportation Engineering, Shenzhen Polytechnic, Shenzhen 518055, P.R. China cDepartment of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, P. R. China dGraduate School of Life Science and Systems Engineering, Kyushu Institute of Technology, Kitakyushu, Fukuoka 808-0196, Japan *E-mail: [email protected] Abstract Layered perovskites have attracted considerable attention in optoelectronic applications due to their excellent electronic properties and stability. In this work, the quasi-2D aurivillius halide perovskites are investigated using density functional theory. The single-layer aurivillius perovskite Ba2PbI6 is predicted to have a direct bandgap of 1.89 eV, which is close to that of the Ruddlesden–Popper perovskite Cs2PbI4. The electronic structures near the Fermi level are mainly governed by the [PbX6] octahedra, which leads to similar electronic properties to that of Cs2PbI4. Decomposition energies reveal that these aurivillius perovskites exhibit thermal instability. Increasing the number of [PbX6] octahedra layer can enhance the stability and reduce the bandgap. Bi- and In-based aurivillius perovskites are also calculated to evaluate the Pb-free alternatives. These calculations can serve as a theoretical support in exploring novel layered perovskites. Introduction Since the first report of organic–inorganic halide perovskite (OIHP) solar cells in 2009, the power conversion efficiency (PCE) has dramatically increased to 25.02%.1–3 OIHP exhibits unprecedented optoelectronic properties, such as high carrier mobility, large optical absorption coefficient, and low-cost fabrication method.4–6 Thus, they are promising absorber materials of next-generation solar cells. -

Metal Oxide Compact Electron Transport Layer Modification For
materials Review Metal Oxide Compact Electron Transport Layer Modification for Efficient and Stable Perovskite Solar Cells Md. Shahiduzzaman 1,* , Shoko Fukaya 2, Ersan Y. Muslih 3, Liangle Wang 2 , Masahiro Nakano 3, Md. Akhtaruzzaman 4, Makoto Karakawa 1,2,3, Kohshin Takahashi 3, Jean-Michel Nunzi 1,5 and Tetsuya Taima 1,2,3,* 1 Nanomaterials Research Institute, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; karakawa@staff.kanazawa-u.ac.jp (M.K.); [email protected] (J.-M.N.) 2 Graduate School of Frontier Science Initiative, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; [email protected] (S.F.); [email protected] (L.W.) 3 Graduate School of Natural Science and Technology, Kanazawa University, Kakuma, Kanazawa 920-1192, Japan; [email protected] (E.Y.M.); [email protected] (M.N.); [email protected] (K.T.) 4 Solar Energy Research Institute, The National University of Malaysia, Bangi 43600, Malaysia; [email protected] 5 Department of Physics, Engineering Physics and Astronomy, Queens University, Kingston, ON K7L-3N6, Canada * Correspondence: [email protected] (M.S.); [email protected] (T.T.); Tel.: +81-76-234-4937 (M.S.) Received: 14 April 2020; Accepted: 9 May 2020; Published: 11 May 2020 Abstract: Perovskite solar cells (PSCs) have appeared as a promising design for next-generation thin-film photovoltaics because of their cost-efficient fabrication processes and excellent optoelectronic properties. However, PSCs containing a metal oxide compact layer (CL) suffer from poor long-term stability and performance. The quality of the underlying substrate strongly influences the growth of the perovskite layer. -

Current–Voltage Characteristics of Manganite–Titanite Perovskite Junctions
Current–voltage characteristics of manganite–titanite perovskite junctions Benedikt Ifland1, Patrick Peretzki2, Birte Kressdorf1, Philipp Saring2, Andreas Kelling1, Michael Seibt2 and Christian Jooss*1 Full Research Paper Open Access Address: Beilstein J. Nanotechnol. 2015, 6, 1467–1484. 1Institute of Materials Physics, University of Goettingen, doi:10.3762/bjnano.6.152 Friedrich-Hund-Platz 1, 37077 Goettingen, Germany and 24th Physical Institute, University of Goettingen, Received: 06 March 2015 Friedrich-Hund-Platz 1, 37077 Goettingen, Germany Accepted: 16 June 2015 Published: 07 July 2015 Email: Christian Jooss* - [email protected] This article is part of the Thematic Series "Materials for sustainable energy production, storage, and conversion". * Corresponding author Guest Editor: M. Fichtner Keywords: current–voltage characteristics; perovskites; photovoltaics; polarons © 2015 Ifland et al; licensee Beilstein-Institut. License and terms: see end of document. Abstract After a general introduction into the Shockley theory of current voltage (J–V) characteristics of inorganic and organic semicon- ductor junctions of different bandwidth, we apply the Shockley theory-based, one diode model to a new type of perovskite junc- tions with polaronic charge carriers. In particular, we studied manganite–titanate p–n heterojunctions made of n-doped SrTi1−yNbyO3, y = 0.002 and p-doped Pr1−xCaxMnO3, x = 0.34 having a strongly correlated electron system. The diffusion length of the polaron carriers was analyzed by electron beam-induced current (EBIC) in a thin cross plane lamella of the junction. In the J–V characteristics, the polaronic nature of the charge carriers is exhibited mainly by the temperature dependence of the micro- scopic parameters, such as the hopping mobility of the series resistance and a colossal electro-resistance (CER) effect in the parallel resistance. -

Perovskite and Pyrochlore Tantalum Oxide Nitrides: Synthesis and Characterization
Perovskite and Pyrochlore Tantalum Oxide Nitrides: Synthesis and Characterization Thesis Presented in Partial Fulfillment of the Requirements for the Degree Master of Science in the Graduate School of The Ohio State University by Spencer Hampton Porter, B.S. Graduate Program in Chemistry Ohio State University 2012 Thesis Committee Dr. Patrick Woodward, Advisor Dr. Joshua Goldberger Copyright Spencer Porter 2012 Abstract Oxide nitrides are an emerging class of compounds. Perovskite RETaN2O [RE = La (Imma), Ce (Pnma), Pr (Pnma)] as well as ATaO2N[A = Ca (Pnma), Sr (I 4/mcm), Ba (Pm3¯m)] and pyrochlore RE 2Ta2N2O5 where RE = Ce, Pr (both: Fd3¯m) have been synthesized by solid state and solution-based methods. Crystal structures solved by powder XRD and NPD for all the rare earth analogs (La, Ce, Pr) are reported for the first time. Studies on the preparation techniques of oxide nitrides in both, bulk powder and film format, has shown that solution based precipitation tech- niques decrease crystallite size, increase reactivity, and enable isomorphic films by sedimentation processes. Computational studies on anion ordering generated a library of ordering models herein and finds that, like O2N com- pounds, an ordered cis orientation and out-of-center tantalum displacement provide the most stable model for perovskites with an -N2O anion stoi- chiometry. UV-Vis diffuse reflectance reveals band gaps for CeTaN2O (2.0 eV), PrTaN2O (2.0 eV), Ce2Ta2N2O5 (3.0 eV) and Pr2Ta2N2O5 (3.3 eV). Structure-property relations from calculations elucidate that valence band maximum positions within the compound series is affected by bond lengths and f-orbital contributions. -

Investigations Into the Structure and Properties of Ordered Perovskites, Layered Perovskites, and Defect Pyrochlores
INVESTIGATIONS INTO THE STRUCTURE AND PROPERTIES OF ORDERED PEROVSKITES, LAYERED PEROVSKITES, AND DEFECT PYROCHLORES. DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Meghan C. Knapp ***** The Ohio State University 2006 Dissertation Committee: Approved by Professor Patrick M. Woodward, Advisor Professor Claudia Turro _________________________________ Professor Terry Gustafson Advisor Graduate Program in Chemistry Professor Mohit Randeria ABSTRACT The work described in this thesis explores the effects of chemical substitution on the structures and properties of perovskites, layered perovskites, and defect pyrochlores. Layered perovskites, particularly of the variety K2NiF4, the n = 1 Ruddlesden-Popper structure, were studied to determine the factors that drive octahedral tilting distortions. It was determined that these structures, which are more inherently strained than perovskites, are influenced by the bonding environment around the anions and the A-cation as well as the electrostatic interactions between layers. The effects of cation ordering on the symmetry of Ruddlesden-Popper structures are also presented. Dion-Jacobson structures were also analyzed, and it was found that the trends that govern the behavior of Ruddlesden-Popper structures were not applicable. When n = 1 for Dion-Jacobson structures, the weak inter-layer interactions make the parent structure prone to tilting and plane slippage. This stoichiometry has several competing structures, many of which are observed for AMO4 compounds with highly covalent M-O interactions. Stoichiometric perovskites with multiple A-cations rarely exhibit layered ordering of the A-cations. Double perovskites having two A-cations and two M-cations with the formula AA'MM'O6 (A= Na, K, Li, A' = La, M = Mg, Sc M' = W, Nb, Sb, Te, or when M = M', M = Ti, Zr) were studied to determine the driving force for layered ordering of A-site cations. -

Tin Titanate – the Hunt for a New Ferroelectric Perovskite
Tin Titanate – the hunt for a new ferroelectric perovskite J. Gardner,1 Atul Thakre,2,3 Ashok Kumar,2,3 J. F. Scott,1,4 1 School of Chemistry, University of St. Andrews, St. Andrews, KY16 9ST, United Kingdom 2 CSIR-National Physical Laboratory (CSIR-NPL), Dr. K. S. Krishnan Road, Delhi 110012, India 3 Academy of Scientific and Innovative Research (AcSIR), CSIR-NPL Campus, Dr. K. S. Krishnan Road, Delhi 110012, India 4 School of Physics and Astronomy, University of St. Andrews, St. Andrews, Fife KY16 9SS, United Kingdom Email: [email protected] and [email protected] Abstract We review all the published literature and show that there is no experimental evidence for homogeneous tin titanate SnTiO3 in bulk or thin-film form. Instead a combination of unrelated artefacts are easily misinterpreted. The X-ray Bragg data are contaminated by double scattering from the Si substrate, giving a strong line at the 2-theta angle exactly where perovskite SnTiO3 should appear. The strong dielectric divergence near 560K is irreversible and arises from oxygen site detrapping, accompanied by Warburg/Randles interfacial anomalies. The small (4 uC/cm2) apparent ferroelectric hysteresis remains in samples shown in pure (Sn,Ti)O2 rutile/cassiterite, in which ferroelectricity is forbidden. Only very recent German work reveals real bulk SnTiO3, but this is completely inhomogeneous, consisting of an elaborate array of stacking faults, not suitable for ferroelectric devices. Unpublished TEM data reveal an inhomogeneous SnO layered structured thin films, related to shell-core structures. The harsh conclusion is that there is a combination of unrelated artefacts masquerading as ferroelectricity in powders and ALD films; and only a trace of a second phase in Cambridge PLD data suggests any perovskite content at all.