Major, Trace, and Rare-Earth Element Geochemistry of Nb-V Rich
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of the Mineral Information Package for the Khanneshin Carbonatite Area of Interest
Chapter 21A. Summary of the Mineral Information Package for the Khanneshin Carbonatite Area of Interest Contribution by Robert D. Tucker, Harvey E. Belkin, Klaus J. Schulz, Stephen G. Peters, and Kim P. Buttleman Abstract The Khanneshin carbonatite is a deeply dissected igneous complex of Quaternary age that rises approximately 700 meters above the flat-lying Neogene sediments of the Registan Desert, Helmand Province, Afghanistan. The complex consists almost exclusively of carbonate-rich intrusive and extrusive igneous rocks, crudely circular in outline, with only three small hypabyssal plugs of leucite phonolite and leucitite outcropping in the southeastern part of the complex. The complex is broadly divisible into a central intrusive vent (or massif), approximately 4 kilometers in diameter, consisting of coarse-grained sövite and brecciated and agglomeratic barite-ankerite alvikite; a thin marginal zone (less than 1 kilometer wide) of outwardly dipping (5°–45°). Neogene sedimentary strata; and a peripheral apron of volcanic and volcaniclastic strata extending another 3–5 kilometers away from the central intrusive massif. Small satellitic intrusions of biotite-calcite carbonatite, no larger than 400 meters in diameter, crop out on the southern and southeastern margin of the central intrusive massif. In the 1970s several teams of Soviet geologists identified prospective areas of interest for uranium, phosphorus, and light rare earth element (LREE) mineralization in four regions of the carbonatite complex. High uranium concentrations are reported in two regions; the greatest concentrations are confined to silicified shear zones in sandy clay approximately 1.1 kilometers southwest of the peripheral part of the central vent. An area of phosphorus enrichment, primarily occurring in apatite, is present in coarse-grained agglomeratic alvikite, with abundant fenite xenoliths, approximately 750 meters south of the periphery of the central vent. -

The Hydrous Component in Andradite Garnet
American Mineralogist, Volume 83, pages 835±840, 1998 The hydrous component in andradite garnet GEORG AMTHAUER* AND GEORGE R. ROSSMAN² Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. ABSTRACT Twenty-two andradite samples from a variety of geological environments and two syn- thetic hydroandradite samples were studied by Fourier transform IR spectroscopy. Their 2 spectra show that H enters andradite in the form of OH . Amounts up to 6 wt% H2O occur in these samples; those from low-temperature formations contain the most OH2. Some 42 ↔ 42 features in the absorption spectra indicate the hydrogarnet substitution (SiO4) (O4H4) whereas others indicate additional types of OH2 incorporation. The complexity of the spectra due to multi-site distribution of OH2 increases with increasing complexity of the garnet composition. 42 ↔ 42 INTRODUCTION tution (O4H4) (SiO4) . This observation has been Systematic studies have shown that hydroxide is a con®rmed by XRD of a hydrous andradite with a Si de- common minor component of grossular and pyrope-al- ®ciency of about 50%, and a high OH content (Arm- mandine-spessartite garnets (Aines and Rossman 1985; bruster 1995). The structure of this particular sample with Rossman and Aines 1991). Comparable surveys of an- space group Ia3d is composed of disordered microdo- dradite garnet have not been previously presented. Sev- mains containing (SiO4) and (O4H4) tetrahedral units. eral reports indicate that appreciable amounts of OH2 can The aim of the present investigation was to perform a be incorporated in both natural and synthetic andradite- Fourier transform infrared (FTIR) study on different sam- rich garnet (Flint et al. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
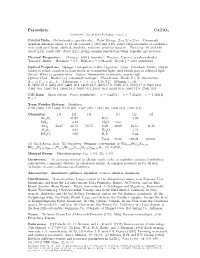
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Nd, Pb and Sr Isotopic Data from the Napak Carbonatite-Nephelinite Centre, Eastern Uganda: an Example of Open-System Crystal Fractionation
Contrib Mineral Petrol (1994) 115:356-366 Contributions tO Mineralogy and Petrology Springer-Verlag 1994 Nd, Pb and Sr isotopic data from the Napak carbonatite-nephelinite centre, eastern Uganda: an example of open-system crystal fractionation Antonio Simonetti and Keith Bell Ottawa-Carleton Geoscience Centre, Department of Earth Sciences, Carleton University, Ottawa, Ontario K1S 5B6, Canada Received June 30, 1992 / Accepted May 25, 1993 Abstract. Nd, Pb and Sr isotopic data from nephelinite magmas cannot be in equilibrium with a lherzolitic mantle lavas from the Tertiary nephelinite-carbonatite complex source (Bultitude and Green 1968, 1971; Allen et al. 1975; of Napak, eastern Uganda, show large isotopic variations Merrill and Wyllie 1975), but are probably the products of that can only be attributed to open-system behaviour. small ( < 5%) degrees of partial melting of a carbonated Possible explanations of the data include mixing between (high C02/H20 ratio) peridotite or pyrolite at high pres- nephelinitic melts derived from an isotopically heterogen- sures (Brey and Green 1977; Brey 1978; Olafsson and eous mantle, or interaction between a HIMU melt and Eggler 1983; Wallace and Green 1988). Experimental re- mafic granulites. In both models crystal fractionation, sults are consistent with derivation of a primary involving olivine and clinopyroxene, played an important nephelinitic liquid from an amphibole peridotite at pres- role. Major element chemistry, textural evidence and iso- sures of 20 to 25 kbar (Olafsson and Eggler 1983; Eggler topic data from clinopyroxene phenocrysts from the ol- 1989). ivine-bearing nephelinites, suggest that the pyroxenes did The eastern branch of the East African Rift Valley not crystallize from their host liquids. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

Mineral Collecting Sites in North Carolina by W
.'.' .., Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie RUTILE GUMMITE IN GARNET RUBY CORUNDUM GOLD TORBERNITE GARNET IN MICA ANATASE RUTILE AJTUNITE AND TORBERNITE THULITE AND PYRITE MONAZITE EMERALD CUPRITE SMOKY QUARTZ ZIRCON TORBERNITE ~/ UBRAR'l USE ONLV ,~O NOT REMOVE. fROM LIBRARY N. C. GEOLOGICAL SUHVEY Information Circular 24 Mineral Collecting Sites in North Carolina By W. F. Wilson and B. J. McKenzie Raleigh 1978 Second Printing 1980. Additional copies of this publication may be obtained from: North CarOlina Department of Natural Resources and Community Development Geological Survey Section P. O. Box 27687 ~ Raleigh. N. C. 27611 1823 --~- GEOLOGICAL SURVEY SECTION The Geological Survey Section shall, by law"...make such exami nation, survey, and mapping of the geology, mineralogy, and topo graphy of the state, including their industrial and economic utilization as it may consider necessary." In carrying out its duties under this law, the section promotes the wise conservation and use of mineral resources by industry, commerce, agriculture, and other governmental agencies for the general welfare of the citizens of North Carolina. The Section conducts a number of basic and applied research projects in environmental resource planning, mineral resource explora tion, mineral statistics, and systematic geologic mapping. Services constitute a major portion ofthe Sections's activities and include identi fying rock and mineral samples submitted by the citizens of the state and providing consulting services and specially prepared reports to other agencies that require geological information. The Geological Survey Section publishes results of research in a series of Bulletins, Economic Papers, Information Circulars, Educa tional Series, Geologic Maps, and Special Publications. -

Mineralogy, Geochemistry and Petrology of a Pyrochlore-Bearing Carbonatite at Seabrook Lake, Ontario
Mineralogy, Geochemistry and Petrology of a Pyrochlore-bearing Carbonatite at Seabrook Lake, Ontario by Myron John Osateriko B.Sc, University of British Columbia, 1965 A Thesis Submitted in Partial Fulfilment ' of the Requirements for the Degree of MASTER OF SCIENCE in the Department of GEOLOGY We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA April, 1967 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that th3 Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Depa rtment The University of British Columbia Vancouver 8, Canada i ABSTRACT The Seabrook Lake carbonatite complex is one of the smallest of nine known carbonatite complexes in central Ontario. The complex, which is one-half square mile in area and pear- shaped in plan, consists of fenitized granite and breccia, mafic breccia, ijolite and related breccia, and carbonatite. The bulbous northern part of the complex consists of a plug-like core of carbonatite surrounded by mafic breccia and carbonatite dykes. The narrow southern part consists of ijolite and related breccia. Enveloping all of these rocks.is a fenitized aureole which grades outward to unaltered granite that underlies much of the surrounding area. The carbonatite is composed of calcite with the following minor mineral, in decreasing order of abundance: goethite, microcline, magnesioriebeckite-riebeckite, magnetite-ulvospinel, apatite, hematite, pyrite, albite, biotite, chlorite, pyrochlore, brookite, sphene, ferroan dolomite (ankerite?), aegirine, chalcopyrite, wollastonite and quartz. -

Geology of the Cluggerfluorite Deposit
Revista Brasileira de Geociências 17(3): 288-294, setembro de 1987 GEOLOGY OF THE CLUGGE RFLUORITE DEPOSIT. MATO PRETO. PARANÁ. BRAZIL ROBERT E. JENK INS n- ABSTRACT The Mato Preto fluorite deposite are located in the Ribeira River valley 80 km NNE from Curitiba, Paraná, Brazil. They consist of three known orebodies and numerous minar occurrences wi thin an area of about 15 km ê. Clugger, the largest dcposit, is a hydrothermnl replacemcnt and fracture-fil ling type in a brecciated and sheared contact zone among carbonatitc, nephellne syenitc, phonolite, and tin guaite of the Cretaceous-Paleocene age Mato Preto Igneous Complex. Fluorite occurs LS four subparallel but coalescing ore lenses which forro envelopes about dikes of phonolite-tinguaite and have strlke lengths of 250 m, aggregate thickness up to 80 m, and extend to at least 120 m in depth . Dikes and Ienses strike N50-55E and dip steeply to the northwest. Fluorite forms matrix replacemcnts and crosscutting veins in premineral and intermineral breccias adjacent to dikes. Fluorite mineralizatioo has been introduced in at least two pulses and is associated with barite-celestlre, apatite, rare earth minerais, and sulfides. Late explosive venting of the system has formed pipes of volcanic breccia with crackle breccia in ore adjacent. Fluorite along breakage planes is recrystallized into monomineralic veinlets. Mineralization is associated with fracture controlled epidote aJteration, but major silicification apparently predates ore. The c1ugger deposit was probably fonne d at low pressures and temperatures, possibly within a vent of a now-eroded Mato Preto volcano. RESUMO Os depósitos de Iluorita de Mato Preto situam-se no vale do Rio Ribeira, 80 km a NNE de Curitiba,Paraná.