High-Pressure Crystal Chemistry of Andradite and Pyrope: Revised
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structure and Physical Properties of Hydrogrossular Mineral Series
STRUCTURE AND PHYSICAL PROPERTIES OF HYDROGROSSULAR MINERAL SERIES A THESIS IN PHYSICS Presented to the Faculty of the University of Missouri-Kansas City in Partial requirements for the degree MASTER OF SCIENCE By PUJA ADHIKARI M.Sc, Tribhuban University, Kathmandu, Nepal, 2010 Kansas City, Missouri 2015 ©2015 PUJA ADHIKARI ALL RIGHTS RESERVED Structure and physical properties of Hydrogrossular mineral series Puja Adhikari, Candidate for the Master of Science Degree University of Missouri-Kansas City, 2015 ABSTRACT The mineral hydrogrossular series (Ca3Al2(SiO4)3-x(OH) 4x; 0 ≤ x ≤ 3) are important water bearing minerals found in the upper and lower part of the Earth’s mantle. They are vital to the planet’s hydrosphere under different hydrothermal conditions. The composition and structure of this mineral series are important in geoscience and share many commonalities with cement and clay materials. Other than the end members of the series x = 0 (grossular) and x = 3 (katoite) which have a cubic garnet structure, the structure of the series is totally unknown. We used large-scale ab initio modeling to investigate the structures and properties for hydrogrossular series for x = 0, 0.5, 1, 1.5, 2, 2.5, 3. Results indicate that for x > 0 and x < 3, the structures are tetragonal. This shows that there is structural change related to the lowering of overall symmetry associated with the composition of SiO4 tetrahedra and AlO6 octahedra. Total Bond order also explains the reason behind the change in the compressibility of the series. The electronic structure, mechanical and optical properties of the hydrogrossular series are calculated and the results for grossular and katoite are in good agreement with the available experimental data. -

The Hydrous Component in Andradite Garnet
American Mineralogist, Volume 83, pages 835±840, 1998 The hydrous component in andradite garnet GEORG AMTHAUER* AND GEORGE R. ROSSMAN² Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. ABSTRACT Twenty-two andradite samples from a variety of geological environments and two syn- thetic hydroandradite samples were studied by Fourier transform IR spectroscopy. Their 2 spectra show that H enters andradite in the form of OH . Amounts up to 6 wt% H2O occur in these samples; those from low-temperature formations contain the most OH2. Some 42 ↔ 42 features in the absorption spectra indicate the hydrogarnet substitution (SiO4) (O4H4) whereas others indicate additional types of OH2 incorporation. The complexity of the spectra due to multi-site distribution of OH2 increases with increasing complexity of the garnet composition. 42 ↔ 42 INTRODUCTION tution (O4H4) (SiO4) . This observation has been Systematic studies have shown that hydroxide is a con®rmed by XRD of a hydrous andradite with a Si de- common minor component of grossular and pyrope-al- ®ciency of about 50%, and a high OH content (Arm- mandine-spessartite garnets (Aines and Rossman 1985; bruster 1995). The structure of this particular sample with Rossman and Aines 1991). Comparable surveys of an- space group Ia3d is composed of disordered microdo- dradite garnet have not been previously presented. Sev- mains containing (SiO4) and (O4H4) tetrahedral units. eral reports indicate that appreciable amounts of OH2 can The aim of the present investigation was to perform a be incorporated in both natural and synthetic andradite- Fourier transform infrared (FTIR) study on different sam- rich garnet (Flint et al. -

Geology Club Mineral: Collecting Trip
Geology Club: Mineral Collecting Trip (10 October 2009) Trip Notes by Charles Merguerian STOP 1 – Grossular Garnet Locality, West Redding, Connecticut. [UTM Coordinates: 630.71E / 4575.38N, Bethel quadrangle]. Covering roughly 60 acres of land, this enigmatic massive fine-grained grossularite garnet + diopside rock in West Redding has made many mineral collectors and geologists take notice. Walk up the steep slope east of Simpaug Turnpike to see highly fractured, massive cinnamon-colored grossular garnet rock, part of a 0.6-km wide heart-shaped mass found at the faulted contact between the Stockbridge Marble (OCs) and injected muscovitic schist of the Rowe Schist member (OCr) of the Hartland Formation (Figure 1). According to Rodgers et al. (1985), we are very near Cameron’s Line (red and black line in Figure 1). Figure 1 – Geologic map of the area surrounding Stop 1 showing the Proterozoic gneissic rocks (Yg) and Cambrian Dalton Schist (Cd) to the west, the Stockbridge Marble (OCs), Cameron’s Line (CL in red), the injected schistose rocks of the Rowe Formation (OCr), and an Ordovician granitoid (Og) that may be responsible for this unusual Ca++-enriched skarn deposit. Note the NW-trending high-angle brittle faults that cut the region. (Adapted from Rodgers et al. 1985.) Two knolls at this locality are almost entirely composed of grossularite garnet (var. essonite) and lesser clinopyroxene. Mostly the garnet occurs alone with minor quartz and localized quartz veining has been observed. Chemical analysis of the garnet (SiO2 = 39.10%, CaO = 34.85%, Al2O3 = 19.61%, and total FeO+Fe2O3 = 5.44%), are quite similar to published analyses of grossular garnet, including the phenomenal grossular garnet crystals from Morelos, Mexico. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Fluorian Garnets from the Host Rocks of the Skaergaard Intrusion: Implications for Metamorphic Fluid Composition
American Mineralogist, Volume 75, pages 859-873,1990 Fluorian garnets from the host rocks of the Skaergaard intrusion: Implications for metamorphic fluid composition CRAIG E. MANNING, * DENNIS K. BIRD Department of Geology, Stanford University, Stanford, California 94305, U.S.A. ABSTRACT Zoned, silica-deficient, calcic garnets containing up to 5 mol% F substitution for 0 formed during contact metamorphism of basalts by the Skaergaard intrusion in East Greenland. Fluorian calcic garnets occur as a retrograde alteration of prograde wollastonite and clinopyroxene that fills vesicles and vugs in lavas 30-70 m from the intrusion. Para- genetically equivalent phases include fluorite, wollastonite, calcic clinopyroxene, prehnite, quartz, and calcite. Electron microprobe analysis shows that the garnets are :::-:93mol% grossular-andradite (grandite) solid solutions and that the F content does not fully com- pensate for the silica deficiency, suggesting the presence of a hydrous component in the garnets. The garnets display discontinuous zoning with respect to Al and Fe, and increases in F, calculated OH, and the Si deficiency with increasing Al concentration are observed. The garnets formed at temperatures between 200 and 420°C based on the coexisting mineral assemblage, and stratigraphic reconstructions indicate pressures of ~ 1 kbar. Assessment of isobaric, isothermal phase relations in the system CaO-A1203-Si02-H20- HF allows estimation of fluid characteristics in equilibrium with fluorian grandite-bearing assemblages. The presence of fluorite, wollastonite, quartz, and calcite with these garnets combined with fluid inclusion data require that the activity (a) of H20 was ~ 1 and that aH.aF was 10-105 to 10-10.0in the coexisting hydrothermal solutions at 200-420 °C and 1 kbar. -

The Effect of Reduced Activity of Anorthite on the Reaction Grossular
AmericanMineralogist, Volume61, pages 889-896,1976 The effectof reducedactivity of anorthiteon thereaction grossular * quartz : anorthite* wollastonite:a modelfor plagioclasein the earth'slower crust anh upper mantle K. E. WrNnoMr AND A. L. BoerrcHrnl Department of Geosciences,The Pennsyluania State (lniuersity Uniuersity Park, Pennsyluania 16802 Abstract The reaction CasAlrSiaor, + SiO, : CaAlzSiros + 2 CaSiOg (erossular) (quartz) (anortbite) (wollastoDite) wasinvestigated at l10ooC,using intermediate-composition plagioclase to determinethe shift of the equilibriumpressure as a functionof compositionat pressureand temperaturecondi- tions representativeof the earth'slower crustand uppermantle. For compositionswithin the rangeAnro-,00, activity of the anorthitecomponent is equalto its molefraction. For composi- tions more albitic than about Anro,the activity of the anorthitecomponent equals its mole fractionmultiplied by a constantfactor of 1.2.This latter behavioris interpretedas a resultof anorthite-likedomains being in an energeticallyunfavorable environment for thesecomposi- tions. Applicationof the data obtainedfrom subsolidusexperiments to meltingbehavior indicates that plagioclaseliquids do not obey Raoult'sLaw, but havean activitycoefficient similar to crystallineplagioclase. This hypothesisis supportedby high-pressuremelting experiments for both anhydrousand hydroussystems. In parts of the lower crustcomposed of basicrock (e.g.gabbro), the calcicplagioclase will be consumedat relativelyshallow levels and over a narrow (2-3 km) -

CRYSTAL SRUKTUR of CALCIUM TITANATE (Catio3) PHOSPHOR DOPED with PRASEODYMIUM and ALUMINIUM IONS
CRYSTAL SRUKTUR OF CALCIUM TITANATE (CaTiO3) PHOSPHOR DOPED WITH PRASEODYMIUM AND ALUMINIUM IONS STRUKTUR KRISTAL FOSFOR KALSIUM TITANIA DIDOPKAN DENGAN ION PRASEODYMIUM DAN ALUMINIUM IONS Siti Aishah Ahmad Fuzi1* and Rosli Hussin2 1 Material Technology Group, Industrial Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia. 2 Department of Physics, Faculty of Science, Universiti Teknologi Malaysia, 81310 Skudai, Johor 1*[email protected], [email protected] Abstract The past three decades have witnessed rapid growth in research and development of luminescence phenomenon because of their diversity in applications. In this paper, Calcium Titanate (CaTiO3) was studied to find a new host material with desirable structural properties for luminescence-based applications. Solid state reactions o 3+ methods were used to synthesis CaTiO3 at 1000 C for 6 hours. Crystal structure of CaTiO3 co-doped with Pr 3+ and Al were investigated using X-Ray Diffraction (XRD) method. Optimum percentage to synthesis CaTiO3 was 3+ obtained at 40 mol%CaO-60 mol%TiO2 with a single doping of 1 mol%Pr . However, a crystal structure of 4 mol% of Al3+ co-doped with Pr3+ was determined as an optimum parameter which suitable for display imaging. Keywords: calcium titanate, anatase, rutile Abstrak Semenjak tiga dekad yang lalu telah menunjukkan peningkatan yang ketara bagi kajian dan pembangunan dalam bidang fotolumiscen. Peningkatan ini berkembang dengan meluas disebabkan oleh kebolehannya untuk diaplikasikan dalam pelbagai kegunaan harian. Dalam manuskrip ini, kalsium titania (CaTiO3) telah dikaji untuk mencari bahan perumah dengan sifat struktur yang bersesuaian bagi aplikasi luminescen. Tindak balas keadaan o pepejal telah digunakan bagi mensintesis CaTiO3 pada suhu 1000 C selama 6 jam. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
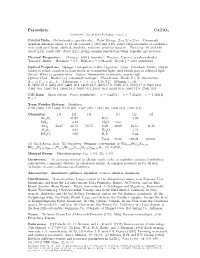
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

The Rutile Deposits of the Eastern United States
THE RUTILE DEPOSITS OF THE EASTERN UNITED STATES. By THOMAS L. WATSON. INTRODUCTION. The titanium-bearing minerals comprise more than 60 distinct species, grouped under a variety of mineral and chemical forms, chiefly as oxides, titanates, titano-silicates, silicates, columbates, and iantalates. These minerals are widely distributed in a variety of associations and in such quantity as to make titanium a relatively abundant element. Clarke* estimates the. amount of titanium in the solid crust of the earth to be 0.44 per cent, equivalent in oxide to 0.73 per cent, the element thus standing in the ninth place in the scale of abundance, next to potassium. Most of the titanium-bearing minerals, however, are rare and are only of scientific interest. The largest concentrations of the element are as oxide (rutile), as iron titanate (ilmenite), and in iron ferrate (magnetite) as intergrown ilmenite. Of these three forms the prin cipal source of the element at present is rutile. The known workable deposits of rutile, however, are extremely few and widely sepa rated, and as the demand for titanium has greatly increased in the last few years it has been necessary for some uses to turn to ilmenite or highly titaniferous magnetites. This paper briefly summarizes present knowledge of the geology of the rutile deposits in the eastern United States and for the sake of comparison discusses several foreign deposits, each of which has produced some rutile. Of the known deposits in the United, States only those in Virginia are of commercial importance. These have been made the subject of a special report 2 by the Virginia Geological Survey, which was preceded by a preliminary paper on the rutile deposits of Amherst and Nelson counties.3 1 Clarke, F. -

The Journal of Gemmology Editor: Dr R.R
he Journa TGemmolog Volume 25 No. 8 October 1997 The Gemmological Association and Gem Testing Laboratory of Great Britain Gemmological Association and Gem Testing Laboratory of Great Britain 27 Greville Street, London Eel N SSU Tel: 0171 404 1134 Fax: 0171 404 8843 e-mail: [email protected] Website: www.gagtl.ac.uklgagtl President: Professor R.A. Howie Vice-Presidents: LM. Bruton, Af'. ram, D.C. Kent, R.K. Mitchell Honorary Fellows: R.A. Howie, R.T. Liddicoat Inr, K. Nassau Honorary Life Members: D.). Callaghan, LA. lobbins, H. Tillander Council of Management: C.R. Cavey, T.]. Davidson, N.W. Decks, R.R. Harding, I. Thomson, V.P. Watson Members' Council: Aj. Allnutt, P. Dwyer-Hickey, R. fuller, l. Greatwood. B. jackson, J. Kessler, j. Monnickendam, L. Music, l.B. Nelson, P.G. Read, R. Shepherd, C.H. VVinter Branch Chairmen: Midlands - C.M. Green, North West - I. Knight, Scottish - B. jackson Examiners: A.j. Allnutt, M.Sc., Ph.D., leA, S.M. Anderson, B.Se. (Hons), I-CA, L. Bartlett, 13.Se, .'vI.phil., I-G/\' DCi\, E.M. Bruton, FGA, DC/\, c.~. Cavey, FGA, S. Coelho, B.Se, I-G,\' DGt\, Prof. A.T. Collins, B.Sc, Ph.D, A.G. Good, FGA, f1GA, Cj.E. Halt B.Sc. (Hons), FGr\, G.M. Howe, FG,'\, oo-, G.H. jones, B.Se, PhD., FCA, M. Newton, B.Se, D.PhiL, H.L. Plumb, B.Sc., ICA, DCA, R.D. Ross, B.5e, I-GA, DGA, P..A.. Sadler, 13.5c., IGA, DCA, E. Stern, I'GA, DC/\, Prof. I.