Structures and Relationships of Some Perovskite-Type Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symmetry of Fulleroids Stanislav Jendrol’, František Kardoš
Symmetry of Fulleroids Stanislav Jendrol’, František Kardoš To cite this version: Stanislav Jendrol’, František Kardoš. Symmetry of Fulleroids. Klaus D. Sattler. Handbook of Nanophysics: Clusters and Fullerenes, CRC Press, pp.28-1 - 28-13, 2010, 9781420075557. hal- 00966781 HAL Id: hal-00966781 https://hal.archives-ouvertes.fr/hal-00966781 Submitted on 27 Mar 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Symmetry of Fulleroids Stanislav Jendrol’ and Frantiˇsek Kardoˇs Institute of Mathematics, Faculty of Science, P. J. Saf´arikˇ University, Koˇsice Jesenn´a5, 041 54 Koˇsice, Slovakia +421 908 175 621; +421 904 321 185 [email protected]; [email protected] Contents 1 Symmetry of Fulleroids 2 1.1 Convexpolyhedraandplanargraphs . .... 3 1.2 Polyhedral symmetries and graph automorphisms . ........ 4 1.3 Pointsymmetrygroups. 5 1.4 Localrestrictions ............................... .. 10 1.5 Symmetryoffullerenes . .. 11 1.6 Icosahedralfulleroids . .... 12 1.7 Subgroups of Ih ................................. 15 1.8 Fulleroids with multi-pentagonal faces . ......... 16 1.9 Fulleroids with octahedral, prismatic, or pyramidal symmetry ........ 19 1.10 (5, 7)-fulleroids .................................. 20 1 Chapter 1 Symmetry of Fulleroids One of the important features of the structure of molecules is the presence of symmetry elements. -

Origin of Icosahedral Symmetry in Viruses
Origin of icosahedral symmetry in viruses Roya Zandi†‡, David Reguera†, Robijn F. Bruinsma§, William M. Gelbart†, and Joseph Rudnick§ Departments of †Chemistry and Biochemistry and §Physics and Astronomy, University of California, Los Angeles, CA 90095-1569 Communicated by Howard Reiss, University of California, Los Angeles, CA, August 11, 2004 (received for review February 2, 2004) With few exceptions, the shells (capsids) of sphere-like viruses (11). This problem is in turn related to one posed much earlier have the symmetry of an icosahedron and are composed of coat by Thomson (12), involving the optimal distribution of N proteins (subunits) assembled in special motifs, the T-number mutually repelling points on the surface of a sphere, and to the structures. Although the synthesis of artificial protein cages is a subsequently posed ‘‘covering’’ problem, where one deter- rapidly developing area of materials science, the design criteria for mines the arrangement of N overlapping disks of minimum self-assembled shells that can reproduce the remarkable properties diameter that allow complete coverage of the sphere surface of viral capsids are only beginning to be understood. We present (13). In a similar spirit, a self-assembly phase diagram has been here a minimal model for equilibrium capsid structure, introducing formulated for adhering hard disks (14). These models, which an explicit interaction between protein multimers (capsomers). all deal with identical capsomers, regularly produced capsid Using Monte Carlo simulation we show that the model reproduces symmetries lower than icosahedral. On the other hand, theo- the main structures of viruses in vivo (T-number icosahedra) and retical studies of viral assembly that assume icosahedral sym- important nonicosahedral structures (with octahedral and cubic metry are able to reproduce the CK motifs (15), and, most symmetry) observed in vitro. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

The Cubic Groups
The Cubic Groups Baccalaureate Thesis in Electrical Engineering Author: Supervisor: Sana Zunic Dr. Wolfgang Herfort 0627758 Vienna University of Technology May 13, 2010 Contents 1 Concepts from Algebra 4 1.1 Groups . 4 1.2 Subgroups . 4 1.3 Actions . 5 2 Concepts from Crystallography 6 2.1 Space Groups and their Classification . 6 2.2 Motions in R3 ............................. 8 2.3 Cubic Lattices . 9 2.4 Space Groups with a Cubic Lattice . 10 3 The Octahedral Symmetry Groups 11 3.1 The Elements of O and Oh ..................... 11 3.2 A Presentation of Oh ......................... 14 3.3 The Subgroups of Oh ......................... 14 2 Abstract After introducing basics from (mathematical) crystallography we turn to the description of the octahedral symmetry groups { the symmetry group(s) of a cube. Preface The intention of this account is to provide a description of the octahedral sym- metry groups { symmetry group(s) of the cube. We first give the basic idea (without proofs) of mathematical crystallography, namely that the 219 space groups correspond to the 7 crystal systems. After this we come to describing cubic lattices { such ones that are built from \cubic cells". Finally, among the cubic lattices, we discuss briefly the ones on which O and Oh act. After this we provide lists of the elements and the subgroups of Oh. A presentation of Oh in terms of generators and relations { using the Dynkin diagram B3 is also given. It is our hope that this account is accessible to both { the mathematician and the engineer. The picture on the title page reflects Ha¨uy'sidea of crystal structure [4]. -

Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of F Orbitals
molecules Article Systematics of Atomic Orbital Hybridization of Coordination Polyhedra: Role of f Orbitals R. Bruce King Department of Chemistry, University of Georgia, Athens, GA 30602, USA; [email protected] Academic Editor: Vito Lippolis Received: 4 June 2020; Accepted: 29 June 2020; Published: 8 July 2020 Abstract: The combination of atomic orbitals to form hybrid orbitals of special symmetries can be related to the individual orbital polynomials. Using this approach, 8-orbital cubic hybridization can be shown to be sp3d3f requiring an f orbital, and 12-orbital hexagonal prismatic hybridization can be shown to be sp3d5f2g requiring a g orbital. The twists to convert a cube to a square antiprism and a hexagonal prism to a hexagonal antiprism eliminate the need for the highest nodality orbitals in the resulting hybrids. A trigonal twist of an Oh octahedron into a D3h trigonal prism can involve a gradual change of the pair of d orbitals in the corresponding sp3d2 hybrids. A similar trigonal twist of an Oh cuboctahedron into a D3h anticuboctahedron can likewise involve a gradual change in the three f orbitals in the corresponding sp3d5f3 hybrids. Keywords: coordination polyhedra; hybridization; atomic orbitals; f-block elements 1. Introduction In a series of papers in the 1990s, the author focused on the most favorable coordination polyhedra for sp3dn hybrids, such as those found in transition metal complexes. Such studies included an investigation of distortions from ideal symmetries in relatively symmetrical systems with molecular orbital degeneracies [1] In the ensuing quarter century, interest in actinide chemistry has generated an increasing interest in the involvement of f orbitals in coordination chemistry [2–7]. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
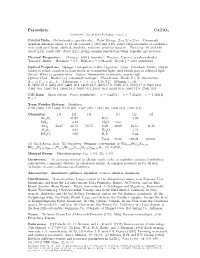
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Can Every Face of a Polyhedron Have Many Sides ?
Can Every Face of a Polyhedron Have Many Sides ? Branko Grünbaum Dedicated to Joe Malkevitch, an old friend and colleague, who was always partial to polyhedra Abstract. The simple question of the title has many different answers, depending on the kinds of faces we are willing to consider, on the types of polyhedra we admit, and on the symmetries we require. Known results and open problems about this topic are presented. The main classes of objects considered here are the following, listed in increasing generality: Faces: convex n-gons, starshaped n-gons, simple n-gons –– for n ≥ 3. Polyhedra (in Euclidean 3-dimensional space): convex polyhedra, starshaped polyhedra, acoptic polyhedra, polyhedra with selfintersections. Symmetry properties of polyhedra P: Isohedron –– all faces of P in one orbit under the group of symmetries of P; monohedron –– all faces of P are mutually congru- ent; ekahedron –– all faces have of P the same number of sides (eka –– Sanskrit for "one"). If the number of sides is k, we shall use (k)-isohedron, (k)-monohedron, and (k)- ekahedron, as appropriate. We shall first describe the results that either can be found in the literature, or ob- tained by slight modifications of these. Then we shall show how two systematic ap- proaches can be used to obtain results that are better –– although in some cases less visu- ally attractive than the old ones. There are many possible combinations of these classes of faces, polyhedra and symmetries, but considerable reductions in their number are possible; we start with one of these, which is well known even if it is hard to give specific references for precisely the assertion of Theorem 1. -

On Polyhedra with Transitivity Properties
Discrete Comput Geom 1:195-199 (1986) iSCrete & Computathm~ ~) 1986 Springer-V©rlagometrvNew York Inc. IF On Polyhedra with Transitivity Properties J. M. Wills Mathematics Institute, University of Siegen, Hoelderlinstrasse 3, D-5900 Siegen, FRG Abstract. In a remarkable paper [4] Griinbaum and Shephard stated that there is no polyhedron of genus g > 0, such that its symmetry group acts transitively on its faces. If this condition is slightly weakened, one obtains" some interesting polyhedra with face-transitivity (resp. vertex-transitivity). In recent years several attempts have been/made to find polyhedral analogues for the Platonic solids in the Euclidean 3-space E 3 (cf. [1, 4, 5, 7-14]). By a polyhedron P we mean the union of a finite number of plane polygons in E 3 which form a 2-manifold without boundary and without self-intersections, where, as usual, a plane polygon is a plane set bounded by finitely many line segments. If the plane polygons are disjoint except for their edges, they are called the faces of P. We require adjacent faces to be non-coplanar. The vertices and edges of P are the vertices and edges of the faces of P. The genus of the underlying 2-manifold is the genus of P. A flag of P is a triplet consisting of one vertex, one edge incident with this vertex, and one face containing this edge. If, for given p- 3, q >- 3 all faces of P are p-gons and all vertices q-valent, we say that P is equivelar, i.e., has equal flags (cf. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy.