Synthesis and Characterization of Yttrium Aluminium Garnet (YAG
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Compact Fluorescent Light Bulbs
Compact Fluorescent Light Bulbs What is a compact fluorescent lamp (CFL) bulb? A CFL bulb is a type of fluorescent bulb that screws into a standard light socket, such as a lamp or ceiling light fixture. CFLs use much less energy and last up to 10 times longer than standard light bulbs. What is in a compact fluorescent lamp (CFL) bulb? A CFL bulb is made of glass, a ceramic and metal base, a luminous powder called phosphor, and a small amount of mercury. How much mercury is contained in a CFL bulb? Manufacturers report that the amount of mercury contained in a CFL bulb is five milligrams, which is less than two ten-thousandths of an ounce. The mercury could be in the form of an invisible vapor or in a bead the size of the period at the end of this sentence. A mercury fever thermometer contains about 100 times more mercury than a CFL bulb. Is it harmful is it to be in the room where a CFL bulb has broken? The amount of mercury vapor that is released from one broken bulb is not enough to make anyone sick. However, it is best to avoid any exposure to mercury. We recommend that you ventilate the room air to the outdoors by opening a window or a door and leave the room for a few hours before cleaning up the broken bulb. How should I clean up a broken CFL bulb? It is not necessary to hire a professional to clean up the bulb. By following the directions below, you can safely clean up a broken CFL bulb. -

High Moisture Accelerated Mechanical Behavior Degradation of Phosphor/Silicone Composites Used in White Light-Emitting Diodes
Article High Moisture Accelerated Mechanical Behavior Degradation of Phosphor/Silicone Composites Used in White Light-Emitting Diodes Jiajie Fan 1,2,3,*, Zhen Wang 1,2, Xunwei Zhang 1, Zhentao Deng 4, Xuejun Fan 5 and Guoqi Zhang 3 1 College of Mechanical and Electrical Engineering, Hohai University, Changzhou 213022, China 2 State Key Lab of Solid State Lighting, Changzhou Institute of Technology Research for Solid State Lighting, Changzhou 213164, China 3 Department of Microelectronics, EEMCS Faculty, Delft University of Technology, 2628 Delft, The Netherlands 4 College of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen 518061, China 5 Department of Mechanical Engineering, Lamar University, Beaumont, TX 77710, USA * Correspondence: [email protected] Received: 12 June 2019; Accepted: 21 July 2019; Published: 31 July 2019 Abstract: In a high-power white light emitting diode (LED) package, the phosphor/silicone composite is typically used for photometric and colorimetric conversions, ultimately producing the white light. However, the phosphor/silicone composite is always exposed under harsh environments with high temperature, high blue light irradiation and high moisture when the LED operates. Therefore, its reliability issue has become one of the critical bottlenecks to improve the lifetime of a high-power white LED package. As the curing process and mechanical behavior of phosphor/silicone composite essentially determine its reliability, this paper firstly uses an in situ viscosity monitoring approach combined with Differential Scanning Calorimetry (DSC) and Fourier Transform Infrared Spectroscopy (FTIR) analysis to explain the curing mechanism of a phosphor/silicone composite by taking the effects of temperature and phosphor mass fraction into consideration. -

Phosphor-Free Ingan White Light Emitting Diodes Using Flip-Chip Technology
Article Phosphor-Free InGaN White Light Emitting Diodes Using Flip-Chip Technology Ying-Chang Li 1, Liann-Be Chang 1,2,3,*, Hou-Jen Chen 1, Chia-Yi Yen 1, Ke-Wei Pan 1, Bohr-Ran Huang 4, Wen-Yu Kuo 4, Lee Chow 5, Dan Zhou 6 and Ewa Popko 7 1 Graduate Institute of Electro-Optical Engineering, Chang Gung University, Taoyuan 333, Taiwan; [email protected] (Y.-C.L.); [email protected] (H.-J.C.); [email protected] (C.-Y.Y.); [email protected] (K.-W.P.) 2 Department of Otolaryngology-Head and Neck Surgery, Chang Gung Memorial Hospital, Taoyuan 333, Taiwan 3 Department of Materials Engineering, Ming Chi University of Technology, New Taipei City 243, Taiwan 4 Graduate Institute of Electro-Optical Engineering, National Taiwan University of Science and Technology, Taipei 10607, Taiwan; [email protected] (B.-R.H.); [email protected] (W.-Y.K.) 5 Department of Physics, University of Central Florida, Orlando, FL 32816, USA; [email protected] 6 Department of Materials Science and Engineering, University of Central Florida, Orlando, FL 32816, USA; [email protected] 7 Institute of Physics, Wroclaw University of Technology, 50-370 Wroclaw, Poland; [email protected] * Correspondence: [email protected]; Tel.: +886-3-211-8800 Academic Editor: Durga Misra Received: 9 February 2017; Accepted: 17 April 2017; Published: 20 April 2017 Abstract: Monolithic phosphor-free two-color gallium nitride (GaN)-based white light emitting diodes (LED) have the potential to replace current phosphor-based GaN white LEDs due to their low cost and long life cycle. -

Determination of Aluminium As Oxide
DETERMINATION OF ALUMINIUM AS OXIDE By William Blum CONTENTS Page I. Introduction 515 II. General principles 516 III. Historical 516 IV. Precipitation of aluminium hydroxide. 518 1. Hydrogen electrode studies 518 (a) The method 518 (b) Apparatus and solutions employed 518 (c) Results of hydrogen electrode experiments 519 (d) Conclusions from hydrogen electrode experiments 520 2. Selection of an indicator for denning the conditions of precipita- '. tion . 522 3. Factors affecting the form of the precipitate 524 4. Precipitation in the presence of iron 525 V. Washing the precipitate . 525 VI. Separation from other elements 526 VII. Ignition and weighing of the precipitate 528 1. Hygroscopicity of aluminium oxide 529 2. Temperature and time of ignition 529 3. Effect of ammonium chloride upon the ignition 531 VIII. Procedure recommended 532 IX. Confirmatory experiments 532 X. Conclusions '534 I. INTRODUCTION Although a considerable number of precipitants have been pro- posed for the determination of aluminium, direct precipitation of aluminium hydroxide by means of ammonium hydroxide, fol- lowed by ignition to oxide, is most commonly used, especially if no separation from iron is desired, in which latter case special methods must be employed. While the general principles involved in this determination are extremely simple, it has long been recog- nized that certain precautions in the precipitation, washing, and ignition are necessary if accurate results are to be obtained. While, however, most of these details have been studied and dis- cussed by numerous authors, it is noteworthy that few publica- tions or textbooks have taken account of all the factors. In the 515 ; 516 Bulletin of the Bureau of Standards [Voi.i3 present paper it seems desirable, therefore, to assemble the various recommendations and to consider their basis and their accuracy. -

Perovskites: Crystal Structure, Important Compounds and Properties
Perovskites: crystal structure, important compounds and properties Peng Gao GMF Group Meeting 12,04,2016 Solar energy resource PV instillations Global Power Demand Terrestrial sun light To start • We have to solve the energy problem. • Any technology that has good potential to cut carbon emissions by > 10 % needs to be explored aggressively. • Researchers should not be deterred by the struggles some companies are having. • Someone needs to invest in scaling up promising solar cell technologies. Origin And History of Perovskite compounds Perovskite is calcium titanium oxide or calcium titanate, with the chemical formula CaTiO3. The mineral was discovered by Gustav Rose in 1839 and is named after Russian mineralogist Count Lev Alekseevich Perovski (1792–1856).” All materials with the same crystal structure as CaTiO3, namely ABX3, are termed perovskites: Origin And History of Perovskite compounds Very stable structure, large number of compounds, variety of properties, many practical applications. Key role of the BO6 octahedra in ferromagnetism and ferroelectricity. Extensive formation of solid solutions material optimization by composition control and phase transition engineering. A2+ B4+ O2- Ideal cubic perovskite structure (ABO3) Classification of Perovskite System Perovskite Systems Inorganic Halide Oxide Perovskites Perovskites Alkali-halide Organo-Metal Intrinsic Doped Perovskites Halide Perovskites Perovskites A2Cl(LaNb2)O7 Perovskites 1892: 1st paper on lead halide perovskites Structure deduced 1959: Kongelige Danske Videnskabernes -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

In Situ X-Ray Diffraction Study of Phase Transitions of Fetio3 at High Pressures and Temperatures Using a Large-Volume Press and Synchrotron Radiation
American Mineralogist, Volume 91, pages 120–126, 2006 In situ X-ray diffraction study of phase transitions of FeTiO3 at high pressures and temperatures using a large-volume press and synchrotron radiation LI CHUNG MING,1,* YOUNG-HO KIM,2 T. UCHIDA,3 Y. WANG,3 AND M. RIVERS3 1Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. 2Department of Earth and Environment Science, Gyeongsang National University, Jinju 660-701, Korea 3The University of Chicago, 5640 South Ellis Avenue, Chicago, Illinois 60637, U.S.A. ABSTRACT The phase transformation from ilmenite to perovskite in FeTiO3 was directly observed using synchrotron-based X-ray diffraction and a large-volume press. The perovskite phase is temperature quenchable at 20 GPa and converts into the LiNbO3 phase at pressures below 15 GPa at room tem- perature. The LiNbO3 phase transforms into the ilmenite phase at 10 GPa and 673 K. However, the back-transformation from the ilmenite to the LiNbO3 phase was not observed, thus strongly suggesting that the LiNbO3 phase is not thermodynamically stable but rather a retrogressive phase formed from perovskite during decompression at room temperature. By cycling the pressure up and down at temperatures between 773 and 1023 K, the perovskite- ilmenite transformation could be observed in both directions, thus conÞ rming that perovskite is the true high-pressure phase with respect to the ilmenite phase at lower pressures. The phase boundary of the perovskite-ilmenite transformation thus determined in this study is represented by P (GPa) = 16.0 (±1.4) – 0.0012 (±0.0014) T (K), which is inconsistent with P = 25.2 – 0.01 T (K) reported previously (Syono et al. -
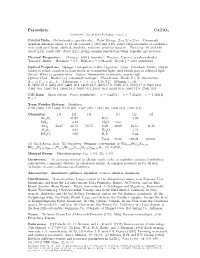
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Scintillation Detectors for X-Rays
INSTITUTE OF PHYSICS PUBLISHING MEASUREMENT SCIENCE AND TECHNOLOGY Meas. Sci. Technol. 17 (2006) R37–R54 doi:10.1088/0957-0233/17/4/R01 REVIEW ARTICLE Scintillation detectors for x-rays Martin Nikl Institute of Physics, Academy of Sciences of the Czech Republic, Cukrovarnicka 10, 162 53 Prague, Czech Republic Received 23 March 2005 Published 10 February 2006 Online at stacks.iop.org/MST/17/R37 Abstract Recent research in the field of phosphor and scintillator materials and related detectors is reviewed. After a historical introduction the fundamental issues are explained regarding the interaction of x-ray radiation with a solid state. Crucial parameters and characteristics important for the performance of these materials in applications, including the employed measurement methods, are described. Extended description of the materials currently in use or under intense study is given. Scintillation detector configurations are further briefly overviewed and selected applications are mentioned in more detail to provide an illustration. Keywords: luminescence intensity, luminescence kinetics, light detection, x-ray detection, scintillators, phosphors, traps and material imperfections (Some figures in this article are in colour only in the electronic version) 1. Introduction development of phosphor and scintillator materials to be used in their exploitation. It was 110 years ago in November 1895 that Wilhelm Conrad It is to be noticed that for registration of x-ray the so- Roentgen noticed the glow of a barium platino-cyanide screen, called direct registration principle is widely used, in which the placed next to his operating discharge tube, and discovered new incoming radiation is directly converted into electrical current invisible and penetrating radiation [1], which was named x-ray in a semiconducting material. -

Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique
Hindawi Advances in Materials Science and Engineering Volume 2019, Article ID 1612456, 7 pages https://doi.org/10.1155/2019/1612456 Research Article Electrocatalytic Properties of Calcium Titanate, Strontium Titanate, and Strontium Calcium Titanate Powders Synthesized by Solution Combustion Technique Oratai Jongprateep ,1,2 Nicha Sato ,1 Ratchatee Techapiesancharoenkij,1,2 and Krissada Surawathanawises1 1Department of Materials Engineering, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand 2Materials Innovation Center, Faculty of Engineering, Kasetsart University, Bangkok 10900, !ailand Correspondence should be addressed to Oratai Jongprateep; [email protected] Received 29 October 2018; Accepted 13 February 2019; Published 4 April 2019 Academic Editor: Alexander Kromka Copyright © 2019 Oratai Jongprateep et al. *is is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Calcium titanate (CaTiO3), strontium titanate (SrTiO3), and strontium calcium titanate (SrxCa1−xTiO3) are widely recognized and utilized as dielectric materials. *eir electrocatalytic properties, however, have not been extensively examined. *e aim of this research is to explore the electrocatalytic performance of calcium titanate, strontium titanate, and strontium calcium titanate, as potential sensing materials. Experimental results revealed that CaTiO3, SrTiO3, and Sr0.5Ca0.5TiO3 powders synthesized by the solution combustion technique consisted of submicrometer-sized particles with 2 specific surface areas ranging from 4.19 to 5.98 m /g. Optical bandgap results indicated that while CaTiO3 and SrTiO3 had bandgap energies close to 3 eV, Sr0.5Ca0.5TiO3 yielded a lower bandgap energy of 2.6 eV. Cyclic voltammetry tests, measured in 0.1 M sodium nitrite, showed oxidation peaks occurring at 0.58 V applied voltage. -

(YAG) Laser Capsulotomy Reference Number: CP.VP.65 Coding Implications Last Review Date: 12/2020 Revision Log
Clinical Policy: Yttrium Aluminium Garnet (YAG) Laser Capsulotomy Reference Number: CP.VP.65 Coding Implications Last Review Date: 12/2020 Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description This policy describes the medical necessity requirements for yttrium aluminium garnet (YAG) laser capsulotomy. Policy/Criteria I. It is the policy of health plans affiliated with Centene Corporation® (Centene) that YAG laser capsulotomy is medically necessary for the following indications: A. Posterior capsular opacification following cataract surgery resulting in best corrected visual acuity of 20/30 or worse associated with symptoms of blurred vision, visual distortion or glare affecting activities of daily living; B. Contraction of the posterior capsule with resulting displacement of the intraocular lens; C. Posterior capsular opacification resulting in best corrected visual acuity of 20/25 or worse, reducing the ability to evaluate and treat retinal detachment. Background YAG capsulotomy is the incision of an opaque posterior lens capsule in an aphakic or pseudophakic eye. This incision allows the capsule to retract and no longer serve as an obstruction to the passage of light through the media to the retina. The incision is performed with YAG laser. The eye examination must confirm the diagnosis of posterior capsular opacification and excludes other ocular causes of functional impairment by one of the following methods: The eye examination should demonstrate decreased light transmission (visual acuity worse than 20/30 or 20/25 if the procedure is performed to assist in the diagnosis and treatment of retinal detachment). Manifest refraction must be recorded with decrease in best-corrected visual acuity. -

The American Mineralogist Journal of the Mineralogical Society of America
THE AMERICAN MINERALOGIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 46 MAY-JUNE, 1961 Nos. 5 and 6 KIMZEYITE, A ZIRCONIUM GARNET FROM MAGNET COVE, ARKANSAS* CHARLES MILTON, BLANCHE L. INGRAM, AND LAWRENCE V. BLADE, U. S. Geological Survey, Washington, D. C. ABSTRACT Kimzeyite, Ca3(Zr, 'Ii, Mg, Fe", Nb)z(AI, Fe"', Si),012, is a new type of garnet oc- curring as dodecahedrons modified by trapezohedron at Magnet Cove, Arkansas, in a carbonatite with abundant apatite, monticellite, calcite, perovskite (dysanalyte), magne- tite, and minor biotite, pyrite, and vesuvianite. It is dark brown, H about 7, isotropic, in- soluble in acids, infusible before the blowpipe, D = 4.0, n = 1.94. The three strongest x-ray powder pattern lines with intensities as measured are 1.667 (10), 2.539 (9), 2.79 (8); the unit cell constant is 12.46 A. Microchemical analysis gave CaO 29.8, Zr02 29.9, Ti203 5.0, MgO 0.5, FeO 0.8, Nb205 1.0, AhO" 11.0, Fe203 13.4, Si02 9.6, sum 101.0 which computes to Cas.n (Zrl.42+4Tio.4o+3Mgo.o7+21'eO.07+2Nbu. 05+;)(Ah.2. +3Feo.98+3Sio. 94+4)012.00. Basically, this is Ca"Zr2(AI,Si)012 with Ti replacing Zr and Fe replacing AI. Zirconium has been found in other garnets (schor]omite from Magnet Cove, melanite from Kaiserstuhl, Germany, and titanian andradite from Oka, Quebec) in quantity up to severa] per cent, but in none of these is it a major constituent. Kimzeyite is named in honor of the Kimzey family, long known in connection with Magnet Cove mineralogy.