Natural Titanite Reference Materials for in Situ U‐Pb and Sm‐Nd Isotopic Measurements by LA‐(MC)‐ICP‐MS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
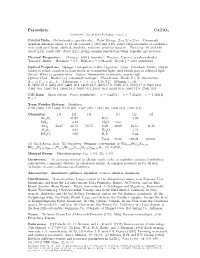
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

Current–Voltage Characteristics of Manganite–Titanite Perovskite Junctions
Current–voltage characteristics of manganite–titanite perovskite junctions Benedikt Ifland1, Patrick Peretzki2, Birte Kressdorf1, Philipp Saring2, Andreas Kelling1, Michael Seibt2 and Christian Jooss*1 Full Research Paper Open Access Address: Beilstein J. Nanotechnol. 2015, 6, 1467–1484. 1Institute of Materials Physics, University of Goettingen, doi:10.3762/bjnano.6.152 Friedrich-Hund-Platz 1, 37077 Goettingen, Germany and 24th Physical Institute, University of Goettingen, Received: 06 March 2015 Friedrich-Hund-Platz 1, 37077 Goettingen, Germany Accepted: 16 June 2015 Published: 07 July 2015 Email: Christian Jooss* - [email protected] This article is part of the Thematic Series "Materials for sustainable energy production, storage, and conversion". * Corresponding author Guest Editor: M. Fichtner Keywords: current–voltage characteristics; perovskites; photovoltaics; polarons © 2015 Ifland et al; licensee Beilstein-Institut. License and terms: see end of document. Abstract After a general introduction into the Shockley theory of current voltage (J–V) characteristics of inorganic and organic semicon- ductor junctions of different bandwidth, we apply the Shockley theory-based, one diode model to a new type of perovskite junc- tions with polaronic charge carriers. In particular, we studied manganite–titanate p–n heterojunctions made of n-doped SrTi1−yNbyO3, y = 0.002 and p-doped Pr1−xCaxMnO3, x = 0.34 having a strongly correlated electron system. The diffusion length of the polaron carriers was analyzed by electron beam-induced current (EBIC) in a thin cross plane lamella of the junction. In the J–V characteristics, the polaronic nature of the charge carriers is exhibited mainly by the temperature dependence of the micro- scopic parameters, such as the hopping mobility of the series resistance and a colossal electro-resistance (CER) effect in the parallel resistance. -

The Propertiesof Anatase Pseudomorphs After
Canadian Mineralogist Vol. 27, pp. 495-498 (1989) THE PROPERTIES OF ANATASE PSEUDOMORPHS AFTER TITANITE ERIC R. VANCE* AND DIANE C. DOERN Atomic Energy of Canada Limited, Whiteshell Nuclear Establishment, Pinawa, Manitoba ROE lLO ABSTRACT The object of the present work was to investigate whether a Ti-rich surface layer tends to protect the Two polycrystalline natural anatase pseudomorphs after titanite against further alteration. This information titanite were studied by scanning electron microscopy, X- is of interest in connection with both the rates of for- ray diffraction and differential thermal and thermogravi- mation of an anatase pseudomorph after titanite, and metric analyses. Millimeter-sized natural crystals of titanite, the long-term leaching of titanite glass-ceramics leached for 30 days in 0.3 or I mol/L HCI in water at (Hayward & Cecchetto 1982, Hayward 1986) 100°C, produced relatively porous pseudomorphs, many of which contain siliceous material and rutile in addition designed for the immobilization of radioactive waste. to anatase. The Ti-rich layer produced in titanite leached in near- neutral ~edia at -90°C is only a few micrometers Keywords: anatase, titanite, pseudomorphs, leaching. thick, even after several months of leaching in the laboratory (Vance 1987). A thicker layer would facili- SOMMAIRE tate the study of protection against leaching, so that anatase pseudomorphs after both natural titanite and Deux echantillons polycristallins d'anatase naturel pro- titanite crystals leached in HCI solutions were exa- duit par Ie remplacement de titanite ont ete etudies en uti- mined. lisant la microscopie electronique a balayage, la diffrac- tion X et des analyses differentielles thermique et thermogravimetrique. -

REVISION 1 Ilmenite Breakdown and Rutile
REVISION 1 Ilmenite breakdown and rutile - titanite stability in metagranitoids: natural observations and experimental results Samuel Angiboust(1,2) and Daniel Harlov(1) (1) Deutsches GeoForschungsZentrum, Telegrafenberg, D-14473 Potsdam, Germany. ([email protected]) (2) Institut de Physique du Globe de Paris, Sorbonne Paris Cité, Univ. Paris Diderot, CNRS, F-75005 Paris, France (*: corresponding autor) [email protected] ABSTRACT Rutile and titanite commonly form by replacement of ilmenite in metamorphic rocks. Exhumed orthogneiss from the W. Alps show that titanite is mostly stable below 1 GPa while rutile seems to dominate within rocks recrystallized under higher pressures. We herein investigate phase relationships for four granitic compositions with variable CaO content at medium to high-pressure conditions (0.7-1.6 GPa, 450-650°C) with a focus on ilmenite breakdown and Ti-bearing species formation. Our piston-cylinder experiments show that, in the investigated P-T range, ilmenite reacts during metamorphism above 1.2-1.4 GPa to form rutile. Below this pressure, titanite is the dominant Ti-bearing species for most granitoid compositions. We also show that the position of this reaction curve is strongly influenced by the whole rock Ca activity. For low Ca activities, rutile may be stable down to 0.7 GPa (and below) within ilmenite pseudomorphs while the titanite stability field may extend to pressures >1.3 GPa for Ca-richer compositions. Both species may be co-stable in one single sample depending on the local Ca activity gradients. The finding of metamorphic rutile within metagranitoids with CaO contents >2 wt% can be considered, under certain conditions, as a reliable indicator of high-pressure metamorphism. -

Minerals Found in Michigan Listed by County
Michigan Minerals Listed by Mineral Name Based on MI DEQ GSD Bulletin 6 “Mineralogy of Michigan” Actinolite, Dickinson, Gogebic, Gratiot, and Anthonyite, Houghton County Marquette counties Anthophyllite, Dickinson, and Marquette counties Aegirinaugite, Marquette County Antigorite, Dickinson, and Marquette counties Aegirine, Marquette County Apatite, Baraga, Dickinson, Houghton, Iron, Albite, Dickinson, Gratiot, Houghton, Keweenaw, Kalkaska, Keweenaw, Marquette, and Monroe and Marquette counties counties Algodonite, Baraga, Houghton, Keweenaw, and Aphrosiderite, Gogebic, Iron, and Marquette Ontonagon counties counties Allanite, Gogebic, Iron, and Marquette counties Apophyllite, Houghton, and Keweenaw counties Almandite, Dickinson, Keweenaw, and Marquette Aragonite, Gogebic, Iron, Jackson, Marquette, and counties Monroe counties Alunite, Iron County Arsenopyrite, Marquette, and Menominee counties Analcite, Houghton, Keweenaw, and Ontonagon counties Atacamite, Houghton, Keweenaw, and Ontonagon counties Anatase, Gratiot, Houghton, Keweenaw, Marquette, and Ontonagon counties Augite, Dickinson, Genesee, Gratiot, Houghton, Iron, Keweenaw, Marquette, and Ontonagon counties Andalusite, Iron, and Marquette counties Awarurite, Marquette County Andesine, Keweenaw County Axinite, Gogebic, and Marquette counties Andradite, Dickinson County Azurite, Dickinson, Keweenaw, Marquette, and Anglesite, Marquette County Ontonagon counties Anhydrite, Bay, Berrien, Gratiot, Houghton, Babingtonite, Keweenaw County Isabella, Kalamazoo, Kent, Keweenaw, Macomb, Manistee, -

Usafri Workshop APS March Meeting Sunday, March 14, 2021
Physics of lower dimensional systems: examples of research made possible through collaborations G. Gebreyesus University of Ghana [email protected] USAfrI Workshop Sponsored by the APS Innovation APS March Meeting Fund Sunday, March 14, 2021 Talk Outline: Low temperature phase of perovskite (ABO3 ) materials: structural, electronic and vibrational properties using extended Hubbard functional Collaboration with Iurii Timrov and Nicola Marzari, EPFL, Switzerland Strontium ruthenate oxides of the Ruddlesden Popper (RP) series, Srn+1 Run O3n+1 (n =1, 2, 3 , ∞): Electronic and magnetic properties using PBEsol and Hubbard functionals Collaboration with Richard Martin, Nicola Seriani, Prosper Ngabonziza, Omololu Akin-Ojo, Jonah Nagura Ideas for Collaborations Low Temperature Phase ABO3 Perovskites using Extended Hubbard functional Outline Structural optimization Collaboration: Electronic properties Prof. Nicola Marzari & Phonon calculations Dr. Iurii Timrov http://theossrv1.epfl. ch/ Low temperature phase Barium Titanite (BaTiO3) Barium Titanite is one of the ABO3 perovskite materials. The atomic positions can be represented: Ba: (0.0 + ∆Ba , 0.0 + ∆Ba , 0.0 + ∆Ba ) Ti: (0.5 + ∆Ti , 0.5 + ∆Ti , 0.5 + ∆Ti ) O1: (0.5 + ∆O1, 0.5 + ∆O1 , 0.0 + ∆O2 ) O2: (0.5 + ∆O1 , 0.0 + ∆O2 , 0.5 + ∆O1 ) O3: (0.0 + ∆O2 , 0.5 + ∆O1 , 0.5 + ∆O1 ) Where ∆Ba, ∆Ti, ∆O1, and ∆O2 are atomic distortions. We consider rhombohedral crystal structure with space group of R3m (160) (point group C3v) and lattice Parameters a = b = c = 4.0036 Å and angle α = β = γ = 89.8390 Kwei G H et. al J.Phys. Chem. 97, 2368, (1993). Self-consistent workflow of DFT+Hubbard atomic localisation & inter-atomic hybridisations I. -

Antiferroelectrics: History, Fundamentals, Crystal Chemistry, Crystal Structures, Size Effects, and Applications
Received: 26 January 2021 | Revised: 19 March 2021 | Accepted: 22 March 2021 DOI: 10.1111/jace.17834 INVITED FEATURE ARTICLE Antiferroelectrics: History, fundamentals, crystal chemistry, crystal structures, size effects, and applications Clive A. Randall1 | Zhongming Fan1 | Ian Reaney2 | Long- Qing Chen1 | Susan Trolier- McKinstry1 1Materials Research Institute and Material Science and Engineering, The Abstract Pennsylvania State University, University Antiferroelectric (AFE) materials are of great interest owing to their scientific rich- Park, PA, USA ness and their utility in high- energy density capacitors. Here, the history of AFEs is 2University of Sheffield, Sheffield, UK reviewed, and the characteristics of antiferroelectricity and the phase transition of an Correspondence AFE material are described. AFEs are energetically close to ferroelectric (FE) phases, Clive A. Randall, Materials Research and thus both the electric field strength and applied stress (pressure) influence the Institute and Material Science and Engineering, The Pennsylvania State nature of the transition. With the comparable energetics between the AFE and FE University, University Park, PA, USA. phases, there can be a competition and frustration of these phases, and either incom- Email: [email protected] mensurate and/or a glassy (relaxor) structures may be observed. The phase transition Funding information in AFEs can also be influenced by the crystal/grain size, particularly at nanometric National Science Foundation, Grant/ dimensions, and may be tuned through the formation of solid solutions. There have Award Number: IIP- 1841453 and been extensive studies on the perovskite family of AFE materials, but many other 1841466 crystal structures host AFE behavior, such as CuBiP2Se6. AFE applications include DC- link capacitors for power electronics, defibrillator capacitors, pulse power de- vices, and electromechanical actuators.