Hydrochloric Acidic Processing of Titanite Ore to Produce a Synthetic Analogue of Korobitsynite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tundrite-(Ce) Na3(Ce; La)4(Ti; Nb)2(Sio4)2(CO3)3O4(OH) ² 2H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Triclinic
Tundrite-(Ce) Na3(Ce; La)4(Ti; Nb)2(SiO4)2(CO3)3O4(OH) ² 2H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Triclinic. Point Group: 1: Crystals acicular along [001] and °attened on 010 , to 3 cm; as stellate groups and spherulitic masses. Twinning: On 010 , producing f g f g pseudorhombohedra. Physical Properties: Cleavage: Pronounced on 010 . Fracture: Splintery. f g Tenacity: Brittle. Hardness = 3 D(meas.) = 3.70{4.12 D(calc.) = 4.06 » Optical Properties: Transparent. Color: Brownish yellow, greenish yellow to bright light green. Streak: Yellowish gray. Luster: Vitreous to adamantine. Optical Class: Biaxial (+). Pleochroism: Weak; X = pale yellow; Z = greenish yellow. Orientation: Z c = 4 {14 . ® = 1.743 ¯ = 1.80 ° = 1.88 2V(meas.) = 76 ^ ± ± ± Cell Data: Space Group: P 1: a = 7.533(4) b = 13.924(6) c = 5.010(2) ® = 99±52(2)0 ¯ = 70±50(3)0 ° = 100± 59(2)0 Z = 1 X-ray Powder Pattern: Il¶³maussaq intrusion, Greenland. 13.49 (100), 2.505 (100), 3.448 (90), 2.766 (90), 3.535 (80), 6.784 (70), 1.914 (70) Chemistry: (1) SiO2 10.03 TiO2 12.20 La2O3 8.57 Ce2O3 24.38 Nd2O3 10.25 RE2O3 5.80 Nb2O5 3.44 CaO 0.75 Na2O 8.20 CO3 16.38 Total [100.00] (1) Il¶³maussaq intrusion, Greenland; by electron microprobe, C con¯rmed by loss on ignition; original analysis given as elements, here recalculated to oxides, corresponding to Na3:17(Ce1:78Nd0:73La0:63RE0:41Ca0:16)§=3:71(Ti1:83Nb0:31)§=2:14(SiO4)2:00(CO3)3:27O4:25: Occurrence: In pegmatite veins associated with nepheline syenites. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

First Terrestrial Occurrence of Titanium
875 Thz Caradian M fuc ralo g is t Vol.35, pp. 875-885(1997) FIRSTTERRESTRIAL OCCURRENCE OF TITANIUM.RICH PYRRHOTITE, MARCASITEAND PVRITEIN A FENITIZEDXENOLITH FROMTHE KHIBINA ALKALINE COMPLEX. RUSSIA ANDREI Y. BARKOVT nxp KAUKO V.O. LAAJOKI Irxtiruteof Geosciencesand Astrorcmy, University of Oula FIN-90570OuIW FinLand YT]RIP.MEN'SHIKOV Geological Instirute, Kola Science Cente, Russian Acadenry of Scimces, 14 Fersman Street, Apatity 1M200 , Russia TUOMO T. AI.APIETI Instituteof Geoscierrcesand Astrotnnry, University of Ouh+FN-90570 OuIu Finlnnl SEPPOJ. SryONEN Instiarcof ElcctronOptics, University of OuhaFN-90570 Ouh+ Finlnnd ABSTRACT The first terrestrial titanium-rich sulfides, the fust natural niobium-rich sulfide FeMgSe, fluorine-rich (ca. 6 wt.Vo D end-memberphlogopite (S().04 wLTo FeO) and ferroan alabandite occurlocally in aheterogeneous xenolit\ enclosed within nepheline syenite in the l(hifiaa alkalins gs6plex, Kola Peninsul4 northwestem Russia- This assemblage is exclusively associated with an alkali-feldspar-rich rock, probably fenite. Associat€d ninerals include corundum, Nb-Zr-bearing rutile and monazite. The maximum Ti content reaches 3.9 w.7o in pynhotite and 2 wt.7o in marcasite and pyrite, which represent prducts of replacement of the pynhotite. Titanium is distributed rather homogeneously within single grains of the pyrrhotite; however, a strong grain-to-gain variation is observed. The Tl-rich sulfides invariably contrin an elevated level ofvanadium (0.2-0 .4 wr.Vo).T\e results indicate that both Ti and V enter into solid solution in the sulfides. Presumably, there is an environmental similarity between the occurrences ofTl-bearing sulfides in trftibina and in meteorites (enstatite chondrites), where Tl-bearing hoilite occurs. -
: Crystal Structure and Revision of Chemical Formula](https://docslib.b-cdn.net/cover/1463/cafetite-ca-ti2o5-h2o-crystal-structure-and-revision-of-chemical-formula-371463.webp)
Cafetite, Ca[Ti2o5](H2O): Crystal Structure and Revision of Chemical Formula
American Mineralogist, Volume 88, pages 424–429, 2003 Cafetite, Ca[Ti2O5](H2O): Crystal structure and revision of chemical formula SERGEY V. K RIVOVICHEV,1,* VICTOR N. YAKOVENCHUK,2 PETER C. BURNS,3 YAKOV A. PAKHOMOVSKY,2 AND YURY P. MENSHIKOV2 1Department of Crystallography, St. Petersburg State University, University Embankment 7/9, St. Petersburg 199034, Russia 2Geological Institute, Kola Science Centre, Russian Academy of Sciences, Fersmana 14, 184200-RU Apatity, Russia 3Department of Civil Engineering and Geological Sciences, University of Notre Dame, Notre Dame, Indiana 46556-0767, U.S.A. ABSTRACT The crystal structure of cafetite, ideally Ca[Ti2O5](H2O), (monoclinic, P21/n, a = 4.9436(15), b = 12.109(4), c = 15.911(5) Å, b = 98.937(5)∞, V = 940.9(5) Å3, Z = 8) has been solved by direct methods and refined to R1 = 0.057 using X-ray diffraction data collected from a crystal pseudo-merohedrally twinned on (001). There are four symmetrically independent Ti cations; each is octahedrally coordi- nated by six O atoms. The coordination polyhedra around the Ti cations are strongly distorted with individual Ti-O bond lengths ranging from 1.743 to 2.223 Å (the average <Ti-O> bond length is 1.98 Å). Two symmetrically independent Ca cations are coordinated by six and eight anions for Ca1 and Ca2, respectively. The structure is based on [Ti2O5] sheets of TiO6 octahedra parallel to (001). The Ca atoms and H2O groups are located between the sheets and link them into a three-dimensional struc- ture. The structural formula of cafetite confirmed by electron microprobe analysis is Ca[Ti2O5](H2O), . -

Third-Generation Synchrotron X-Ray Diffraction of 6- M Crystal of Raite, Na
Proc. Natl. Acad. Sci. USA Vol. 94, pp. 12263–12267, November 1997 Geology Third-generation synchrotron x-ray diffraction of 6-mm crystal of raite, 'Na3Mn3Ti0.25Si8O20(OH)2z10H2O, opens up new chemistry and physics of low-temperature minerals (crystal structureymicrocrystalyphyllosilicate) JOSEPH J. PLUTH*, JOSEPH V. SMITH*†,DMITRY Y. PUSHCHAROVSKY‡,EUGENII I. SEMENOV§,ANDREAS BRAM¶, CHRISTIAN RIEKEL¶,HANS-PETER WEBER¶, AND ROBERT W. BROACHi *Department of Geophysical Sciences, Center for Advanced Radiation Sources, GeologicalySoilyEnvironmental, and Materials Research Science and Engineering Center, 5734 South Ellis Avenue, University of Chicago, Chicago, IL 60637; ‡Department of Geology, Moscow State University, Moscow, 119899, Russia; §Fersman Mineralogical Museum, Russian Academy of Sciences, Moscow, 117071, Russia; ¶European Synchrotron Radiation Facility, BP 220, 38043, Grenoble, France; and UOP Research Center, Des Plaines, IL 60017 Contributed by Joseph V. Smith, September 3, 1997 ABSTRACT The crystal structure of raite was solved and the energy and metal industries, hydrology, and geobiology. refined from data collected at Beamline Insertion Device 13 at Raite lies in the chemical cooling sequence of exotic hyperal- the European Synchrotron Radiation Facility, using a 3 3 3 3 kaline rocks of the Kola Peninsula, Russia, and the 65 mm single crystal. The refined lattice constants of the Monteregian Hills, Canada (2). This hydrated sodium- monoclinic unit cell are a 5 15.1(1) Å; b 5 17.6(1) Å; c 5 manganese silicate extends the already wide range of manga- 5.290(4) Å; b 5 100.5(2)°; space group C2ym. The structure, nese crystal chemistry (3), which includes various complex including all reflections, refined to a final R 5 0.07. -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

New Mineral Names*,†
American Mineralogist, Volume 100, pages 1649–1654, 2015 New Mineral Names*,† DMITRIY I. BELAKOVSKIY1 AND OLIVIER C. GAGNE2 1Fersman Mineralogical Museum, Russian Academy of Sciences, Leninskiy Prospekt 18 korp. 2, Moscow 119071, Russia 2Department of Geological Sciences, University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada IN THIS ISSUE This New Mineral Names has entries for 10 new minerals, including debattistiite, evdokimovite, ferdowsiite, karpovite, kolskyite, markhininite, protochabournéite, raberite, shulamitite, and vendidaite. DEBATTISTIITE* for 795 unique I > 2σ(I) reflections] corner-sharing As(S,Te)3 A. Guastoni, L. Bindi, and F. Nestola (2012) Debattistiite, pyramids form three-membered distorted rings linked by Ag atoms in triangular or distorted tetrahedral coordination. Certain Ag9Hg0.5As6S12Te2, a new Te-bearing sulfosalt from Len- genbach quarry, Binn valley, Switzerland: description and features of that linkage are similar to those in the structures of crystal structure. Mineralogical Magazine, 76(3), 743–750. trechmannite and minerals of pearceite–polybasite group. Of the seven anion positions, one is almost fully occupied by Te (Te0.93S0.07). The Hg atom is in a nearly perfect linear coordination Debattistiite (IMA 2011-098), ideally Ag9Hg0.5As6S12Te2, is a new mineral discovered in the famous for Pb-Cu-Ag-As-Tl with two Te/S atoms. One of five Ag sites and Hg site, which are bearing sulfosalts Lengenbach quarry in the Binn Valley, Valais, very close (separation 1.137 Å), are partially occupied (50%). Switzerland. Debattistiite has been identified in two specimens Thus there is a statistical distribution (50:50) between Hg(Te,S)2 from zone 1 of the quarry in cavities in dolomitic marble with and AgS2(Te,S)2 polyhedra in the structure. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
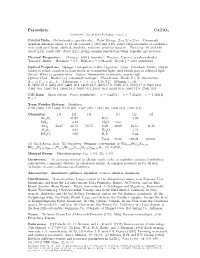
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Petrology of Nepheline Syenite Pegmatites in the Oslo Rift, Norway: Zr and Ti Mineral Assemblages in Miaskitic and Agpaitic Pegmatites in the Larvik Plutonic Complex
MINERALOGIA, 44, No 3-4: 61-98, (2013) DOI: 10.2478/mipo-2013-0007 www.Mineralogia.pl MINERALOGICAL SOCIETY OF POLAND POLSKIE TOWARZYSTWO MINERALOGICZNE __________________________________________________________________________________________________________________________ Original paper Petrology of nepheline syenite pegmatites in the Oslo Rift, Norway: Zr and Ti mineral assemblages in miaskitic and agpaitic pegmatites in the Larvik Plutonic Complex Tom ANDERSEN1*, Muriel ERAMBERT1, Alf Olav LARSEN2, Rune S. SELBEKK3 1 Department of Geosciences, University of Oslo, PO Box 1047 Blindern, N-0316 Oslo Norway; e-mail: [email protected] 2 Statoil ASA, Hydroveien 67, N-3908 Porsgrunn, Norway 3 Natural History Museum, University of Oslo, Sars gate 1, N-0562 Oslo, Norway * Corresponding author Received: December, 2010 Received in revised form: May 15, 2012 Accepted: June 1, 2012 Available online: November 5, 2012 Abstract. Agpaitic nepheline syenites have complex, Na-Ca-Zr-Ti minerals as the main hosts for zirconium and titanium, rather than zircon and titanite, which are characteristic for miaskitic rocks. The transition from a miaskitic to an agpaitic crystallization regime in silica-undersaturated magma has traditionally been related to increasing peralkalinity of the magma, but halogen and water contents are also important parameters. The Larvik Plutonic Complex (LPC) in the Permian Oslo Rift, Norway consists of intrusions of hypersolvus monzonite (larvikite), nepheline monzonite (lardalite) and nepheline syenite. Pegmatites ranging in composition from miaskitic syenite with or without nepheline to mildly agpaitic nepheline syenite are the latest products of magmatic differentiation in the complex. The pegmatites can be grouped in (at least) four distinct suites from their magmatic Ti and Zr silicate mineral assemblages. -

Subject Index, Volume 81, 1996
American Mineralogist, Volume 81, pages 1543-1551, 1996 SUBJECT INDEX, VOLUME 81, 1996 Ag3TeS 1013 geikielite 485 florencite-(La) 1263 4°Ar 940 hornblende 928 glass 229 AuO(OH) 1282 hyttsj6ite 743 granitic melt 202 AuO(OH,Cl)onH20 766 kalsilite 561, 1360 kaolin 26 Achtarandite 516 kaolinite 26 migmatite 141 Afghanite 1003 kinoshitalite 485 orendite 229 Albite 92, 452, 789, 1133, 1344, laumontite 658 peridotite 79 1413 leonhardite 658, 668 rhyolite 158 Alkali feldspar 92, 719, 800, 1425 liandratite 1237 rhyolitic glass 158, 1249 Almandine 418 magnesiochromite 1186 sandstone 213 Altisite 516 magnesite 181 serpentinite 79 Aluminate sodalite 1375 medenbachite 505 volcanic glass 1176 Aluminosilicate glasses 265 muscovite 141, 1460 volcanic rocks 982 Alumoklyuchevskite 249 namuwite 238 Analysis, surface (mineral) Amphibole 135, 495, 1126 nanpingite 105 calcite 1 Analcime 39 nepheline 561, 1360 pyrite 261 Analysis, chemical (mineral) olivine 194, 1519 Anatexis 141 albite 92 omphacite 181 Androsite-(La) 735 alkali feldspar 719 orthopyroxene 676, 842 Ankerite 1141 almandine 418 pentlandite 187 Annite 475 amphibole 135, 495 phlogopite 202, 485,913 Annite-sanidine-magnetite 415 androsite-(La) 735 pigeonite 1166 Anorthoclase 1332 apatite 515 plagioclase 141, 913, 982, 1460 Antimonselite 1013 augite 1166 potassium feldspar 141 Antitaenite 766 bechererite 244 pumpellyite 603 Apatite 864, 1476 betafite 1237 pyralspitic garnet 418 Aragonite 181, 611 biopyribole 404 pyrite 119, 187 Arsenogorceixite 249 biotite 135, 141, 495, 1396, pyrope 418, 706 Asteroid