REVISION 1 Ilmenite Breakdown and Rutile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CRYSTAL SRUKTUR of CALCIUM TITANATE (Catio3) PHOSPHOR DOPED with PRASEODYMIUM and ALUMINIUM IONS
CRYSTAL SRUKTUR OF CALCIUM TITANATE (CaTiO3) PHOSPHOR DOPED WITH PRASEODYMIUM AND ALUMINIUM IONS STRUKTUR KRISTAL FOSFOR KALSIUM TITANIA DIDOPKAN DENGAN ION PRASEODYMIUM DAN ALUMINIUM IONS Siti Aishah Ahmad Fuzi1* and Rosli Hussin2 1 Material Technology Group, Industrial Technology Division, Malaysian Nuclear Agency, Bangi, 43000 Kajang, Selangor Darul Ehsan, Malaysia. 2 Department of Physics, Faculty of Science, Universiti Teknologi Malaysia, 81310 Skudai, Johor 1*[email protected], [email protected] Abstract The past three decades have witnessed rapid growth in research and development of luminescence phenomenon because of their diversity in applications. In this paper, Calcium Titanate (CaTiO3) was studied to find a new host material with desirable structural properties for luminescence-based applications. Solid state reactions o 3+ methods were used to synthesis CaTiO3 at 1000 C for 6 hours. Crystal structure of CaTiO3 co-doped with Pr 3+ and Al were investigated using X-Ray Diffraction (XRD) method. Optimum percentage to synthesis CaTiO3 was 3+ obtained at 40 mol%CaO-60 mol%TiO2 with a single doping of 1 mol%Pr . However, a crystal structure of 4 mol% of Al3+ co-doped with Pr3+ was determined as an optimum parameter which suitable for display imaging. Keywords: calcium titanate, anatase, rutile Abstrak Semenjak tiga dekad yang lalu telah menunjukkan peningkatan yang ketara bagi kajian dan pembangunan dalam bidang fotolumiscen. Peningkatan ini berkembang dengan meluas disebabkan oleh kebolehannya untuk diaplikasikan dalam pelbagai kegunaan harian. Dalam manuskrip ini, kalsium titania (CaTiO3) telah dikaji untuk mencari bahan perumah dengan sifat struktur yang bersesuaian bagi aplikasi luminescen. Tindak balas keadaan o pepejal telah digunakan bagi mensintesis CaTiO3 pada suhu 1000 C selama 6 jam. -

Ultra-High Pressure Aluminous Titanites in Carbonate-Bearing Eclogites at Shuanghe in Dabieshan, Central China
Ultra-high pressure aluminous titanites in carbonate-bearing eclogites at Shuanghe in Dabieshan, central China D. A. CARSWELL Department of Earth Sciences, University of Sheffield, Sheffield $3 7HF, UK R. N. WILSON Department of Geology, University of Leicester, Leicester LE1 7RH, UK AND M. ZtIAI Institute of Geology, Academia Sinica, P.O. Box 634, Beijing 100029, China Abstract Petrographic features and compositions of titanites in eclogites within the ultra-high pressure metamorphic terrane in central Dabieshan are documented and phase equilibria and thermobarometric implications discussed. Carbonate-bearing eclogite pods in marble at Shuanghe contain primary metamorphic aluminous titanites, with up to 39 mol.% Ca(AI,Fe3+)FSiO4 component. These titanites formed as part of a coesite- bearing eclogite assemblage and thus provide the first direct petrographic evidence that AIFTi_IO_j substitution extends the stability of titanite, relative to futile plus carbonate, to pressures within the coesite stability field. However, it is emphasised that A1 and F contents of such titanites do not provide a simple thermobarometric index of P-T conditions but are constrained by the activity of fluorine, relative to CO2, in metamorphic fluids - as signalled by observations of zoning features in these titanites. These ultra-high pressure titanites show unusual breakdown features developed under more H20-rich amphibolite-facies conditions during exhumation of these rocks. In some samples aluminous titanites have been replaced by ilmenite plus amphibole symplectites, in others by symplectitic intergrowths of secondary, lower AI and F, titanite plus plagioclase. Most other coesite-bearing eclogite samples in the central Dabieshan terrane contain peak assemblage rutile often partly replaced by grain clusters of secondary titanites with customary low AI and F contents. -

Occurrence, Alteration Patterns and Compositional Variation of Perovskite in Kimberlites
975 The Canadian Mineralogist Vol. 38, pp. 975-994 (2000) OCCURRENCE, ALTERATION PATTERNS AND COMPOSITIONAL VARIATION OF PEROVSKITE IN KIMBERLITES ANTON R. CHAKHMOURADIAN§ AND ROGER H. MITCHELL Department of Geology, Lakehead University, 955 Oliver Road, Thunder Bay, Ontario P7B 5E1, Canada ABSTRACT The present work summarizes a detailed investigation of perovskite from a representative collection of kimberlites, including samples from over forty localities worldwide. The most common modes of occurrence of perovskite in archetypal kimberlites are discrete crystals set in a serpentine–calcite mesostasis, and reaction-induced rims on earlier-crystallized oxide minerals (typically ferroan geikielite or magnesian ilmenite). Perovskite precipitates later than macrocrystal spinel (aluminous magnesian chromite), and nearly simultaneously with “reaction” Fe-rich spinel (sensu stricto), and groundmass spinels belonging to the magnesian ulvöspinel – magnetite series. In most cases, perovskite crystallization ceases prior to the resorption of groundmass spinels and formation of the atoll rim. During the final evolutionary stages, perovskite commonly becomes unstable and reacts with a CO2- rich fluid. Alteration of perovskite in kimberlites involves resorption, cation leaching and replacement by late-stage minerals, typically TiO2, ilmenite, titanite and calcite. Replacement reactions are believed to take place at temperatures below 350°C, 2+ P < 2 kbar, and over a wide range of a(Mg ) values. Perovskite from kimberlites approaches the ideal formula CaTiO3, and normally contains less than 7 mol.% of other end-members, primarily lueshite (NaNbO3), loparite (Na0.5Ce0.5TiO3), and CeFeO3. Evolutionary trends exhibited by perovskite from most localities are relatively insignificant and typically involve a decrease in REE and Th contents toward the rim (normal pattern of zonation). -

Anorogenic Alkaline Granites from Northeastern Brazil: Major, Trace, and Rare Earth Elements in Magmatic and Metamorphic Biotite and Na-Ma®C Mineralsq
Journal of Asian Earth Sciences 19 (2001) 375±397 www.elsevier.nl/locate/jseaes Anorogenic alkaline granites from northeastern Brazil: major, trace, and rare earth elements in magmatic and metamorphic biotite and Na-ma®c mineralsq J. Pla Cida,*, L.V.S. Nardia, H. ConceicËaÄob, B. Boninc aCurso de PoÂs-GraduacËaÄo em in GeocieÃncias UFRGS. Campus da Agronomia-Inst. de Geoc., Av. Bento GoncËalves, 9500, 91509-900 CEP RS Brazil bCPGG-PPPG/UFBA. Rua Caetano Moura, 123, Instituto de GeocieÃncias-UFBA, CEP- 40210-350, Salvador-BA Brazil cDepartement des Sciences de la Terre, Laboratoire de PeÂtrographie et Volcanologie-Universite Paris-Sud. Centre d'Orsay, Bat. 504, F-91504, Paris, France Accepted 29 August 2000 Abstract The anorogenic, alkaline silica-oversaturated Serra do Meio suite is located within the Riacho do Pontal fold belt, northeast Brazil. This suite, assumed to be Paleoproterozoic in age, encompasses metaluminous and peralkaline granites which have been deformed during the Neoproterozoic collisional event. Preserved late-magmatic to subsolidus amphiboles belong to the riebeckite±arfvedsonite and riebeckite± winchite solid solutions. Riebeckite±winchite is frequently rimmed by Ti±aegirine. Ti-aegirine cores are strongly enriched in Nb, Y, Hf, and REE, which signi®cantly decrease in concentrations towards the rims. REE patterns of Ti-aegirine are strikingly similar to Ti-pyroxenes from the IlõÂmaussaq peralkaline intrusion. Recrystallisation of mineral assemblages was associated with deformation although some original grains are still preserved. Magmatic annite was converted into magnetite and biotite with lower Fe/(Fe 1 Mg) ratios. Recrystallised amphibole is pure riebeckite. Magmatic Ti±Na-bearing pyroxene was converted to low-Ti aegirine 1 titanite ^ astrophyllite/aenigmatite. -
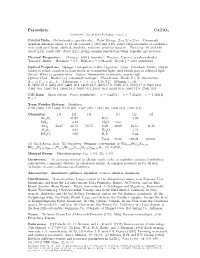
Perovskite Catio3 C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Orthorhombic, Pseudocubic
Perovskite CaTiO3 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic, pseudocubic. Point Group: 2/m 2/m 2/m. Commonly resemble distorted cubes, to 12 cm, striated k [001] and [110], rarely cubo-octahedra or octahedra, with additional forms, skeletal, dendritic; reniform, granular massive. Twinning: 90◦and 180◦ about [101], rarely 180◦ about [121], giving complex penetration twins; lamellar and sectored. Physical Properties: Cleavage: {001}, imperfect. Fracture: Uneven to subconchoidal. Tenacity: Brittle. Hardness = 5.5 D(meas.) = 3.98–4.26 D(calc.) = 4.02 (synthetic). Optical Properties: Opaque, transparent in thin fragments. Color: Iron-black, brown, reddish brown to yellow; colorless to dark brown in transmitted light; dark bluish gray in reflected light. Streak: White to grayish white. Luster: Adamantine to metallic; may be dull. Optical Class: Biaxial (+); commonly isotropic. Pleochroism: Weak; Z > X. Orientation: X = a; Y = c; Z = b. Dispersion: r> v. n= 2.34–2.37 2V(meas.) = 90◦ R: (400) 19.2, (420) 18.8, (440) 18.4, (460) 18.0, (480) 17.6, (500) 17.3, (520) 17.0, (540) 16.8, (560) 16.6, (580) 16.4, (600) 16.2, (620) 16.1, (640) 16.0, (660) 16.0, (680) 15.9, (700) 15.9 Cell Data: Space Group: P nma (synthetic). a = 5.447(1) b = 7.654(1) c = 5.388(1) Z=4 X-ray Powder Pattern: Synthetic. 2.701 (100), 1.911 (50), 2.719 (40), 1.557 (25), 1.563 (16), 3.824 (14), 1.567 (14) Chemistry: (1) (2) (3) (1) (2) (3) Nb2O5 25.99 FeO 5.69 SiO2 0.33 MgO trace TiO2 58.67 38.70 58.75 CaO 40.69 23.51 41.25 Al2O3 0.82 Na2O 1.72 RE2O3 3.08 K2O 0.44 Total 99.36 100.28 100.00 2+ (1) Val d’Aosta, Italy. -

Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis
minerals Article Titanite Ores of the Khibiny Apatite-Nepheline- Deposits: Selective Mining, Processing and Application for Titanosilicate Synthesis Lidia G. Gerasimova 1,2, Anatoly I. Nikolaev 1,2,*, Marina V. Maslova 1,2, Ekaterina S. Shchukina 1,2, Gleb O. Samburov 2, Victor N. Yakovenchuk 1 and Gregory Yu. Ivanyuk 1 1 Nanomaterials Research Centre of Kola Science Centre, Russian Academy of Sciences, 14 Fersman Street, Apatity 184209, Russia; [email protected] (L.G.G.); [email protected] (M.V.M.); [email protected] (E.S.S.); [email protected] (V.N.Y.); [email protected] (G.Y.I.) 2 Tananaev Institute of Chemistry of Kola Science Centre, Russian Academy of Sciences, 26a Fersman Street, Apatity 184209, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-815-557-9231 Received: 4 September 2018; Accepted: 10 October 2018; Published: 12 October 2018 Abstract: Geological setting and mineral composition of (apatite)-nepheline-titanite ore from the Khibiny massif enable selective mining of titanite ore, and its processing with sulfuric-acid method, without preliminary concentration in flotation cells. In this process flow diagram, titanite losses are reduced by an order of magnitude in comparison with a conventional flotation technology. Further, dissolution of titanite in concentrated sulfuric acid produces titanyl sulfate, which, in turn, is a precursor for titanosilicate synthesis. In particular, synthetic analogues of the ivanyukite group minerals, SIV, was synthesized with hydrothermal method from the composition based on titanyl-sulfate, and assayed as a selective cation-exchanger for Cs and Sr. -

The Rutile Deposits of the Eastern United States
THE RUTILE DEPOSITS OF THE EASTERN UNITED STATES. By THOMAS L. WATSON. INTRODUCTION. The titanium-bearing minerals comprise more than 60 distinct species, grouped under a variety of mineral and chemical forms, chiefly as oxides, titanates, titano-silicates, silicates, columbates, and iantalates. These minerals are widely distributed in a variety of associations and in such quantity as to make titanium a relatively abundant element. Clarke* estimates the. amount of titanium in the solid crust of the earth to be 0.44 per cent, equivalent in oxide to 0.73 per cent, the element thus standing in the ninth place in the scale of abundance, next to potassium. Most of the titanium-bearing minerals, however, are rare and are only of scientific interest. The largest concentrations of the element are as oxide (rutile), as iron titanate (ilmenite), and in iron ferrate (magnetite) as intergrown ilmenite. Of these three forms the prin cipal source of the element at present is rutile. The known workable deposits of rutile, however, are extremely few and widely sepa rated, and as the demand for titanium has greatly increased in the last few years it has been necessary for some uses to turn to ilmenite or highly titaniferous magnetites. This paper briefly summarizes present knowledge of the geology of the rutile deposits in the eastern United States and for the sake of comparison discusses several foreign deposits, each of which has produced some rutile. Of the known deposits in the United, States only those in Virginia are of commercial importance. These have been made the subject of a special report 2 by the Virginia Geological Survey, which was preceded by a preliminary paper on the rutile deposits of Amherst and Nelson counties.3 1 Clarke, F. -

Development of Highly Transparent Zirconia Ceramics
11 Development of highly transparent zirconia ceramics Isao Yamashita *1 Masayuki Kudo *1 Koji Tsukuma *1 Highly transparent zirconia ceramics were developed and their optical and mechanical properties were comprehensively studied. A low optical haze value (H<1.0 %), defined as the diffuse transmission divided by the total forward transmission, was achieved by using high-purity powder and a novel sintering process. Theoretical in-line transmission (74 %) was observed from the ultraviolet–visible region up to the infra-red region; an absorption edge was found at 350 nm and 8 µm for the ultraviolet and infrared region, respectively. A colorless sintered body having a high refractive index (n d = 2.23) and a high Abbe’s number (νd = 27.8) was obtained. A remarkably large dielectric constant (ε = 32.7) with low dielectric loss (tanδ = 0.006) was found. Transparent zirconia ceramics are candidates for high-refractive index lenses, optoelectric devices and infrared windows. Transparent zirconia ceramics also possess excellent mechanical properties. Various colored transparent zirconia can be used as exterior components and for complex-shaped gemstones. fabricating transparent cubic zirconia ceramics.9,13-19 1.Introduction Transparent zirconia ceramics using titanium oxide as Transparent and translucent ceramics have been a sintering additive were firstly reported by Tsukuma.15 studied extensively ever since the seminal work on However, the sintered body had poor transparency translucent alumina polycrystal by Coble in the 1960s.1 and low mechanical strength. In this study, highly Subsequently, researchers have conducted many transparent zirconia ceramics of high strength were studies to develop transparent ceramics such as MgO,2 developed. -

Rutile Mineral Chemistry and Zr-In-Rutile Thermometry In
minerals Article Rutile Mineral Chemistry and Zr-in-Rutile Thermometry in Provenance Study of Albian (Uppermost Lower Cretaceous) Terrigenous Quartz Sands and Sandstones in Southern Extra-Carpathian Poland Jakub Kotowski * , Krzysztof Nejbert and Danuta Olszewska-Nejbert Faculty of Geology, University of Warsaw, Zwirki˙ i Wigury 93, 02-089 Warszawa, Poland; [email protected] (K.N.); [email protected] (D.O.-N.) * Correspondence: [email protected] Abstract: The geochemistry of detrital rutile grains, which are extremely resistant to weathering, was used in a provenance study of the transgressive Albian quartz sands in the southern part of extra-Carpathian Poland. Rutile grains were sampled from eight outcrops and four boreholes located on the Miechów, Szydłowiec, and Puławy Segments. The crystallization temperatures of the rutile grains, calculated using a Zr-in-rutile geothermometer, allowed for the division of the study area into three parts: western, central, and eastern. The western group of samples, located in the Citation: Kotowski, J.; Nejbert, K.; Miechów Segment, is characterized by a polymodal distribution of rutile crystallization temperatures ◦ ◦ ◦ Olszewska-Nejbert, D. Rutile Mineral (700–800 C; 550–600 C, and c. 900 C) with a significant predominance of high-temperature forms, Chemistry and Zr-in-Rutile and with a clear prevalence of metapelitic over metamafic rutile. The eastern group of samples, Thermometry in Provenance Study of corresponding to the Lublin Area, is monomodal and their crystallization temperatures peak at Albian (Uppermost Lower 550–600 ◦C. The contents of metapelitic to metamafic rutile in the study area are comparable. The Cretaceous) Terrigenous Quartz central group of rutile samples with bimodal distribution (550–600 ◦C and 850–950 ◦C) most likely Sands and Sandstones in Southern represents a mixing zone, with a visible influence from the western and, to a lesser extent, the eastern Extra-Carpathian Poland. -

Natural Titanite Reference Materials for in Situ U‐Pb and Sm‐Nd Isotopic Measurements by LA‐(MC)‐ICP‐MS
Vol. 43 — N° 3 09 p.355– 384 19 Natural Titanite Reference Materials for In Situ U-Pb and Sm-Nd Isotopic Measurements by LA-(MC)-ICP-MS Qian Ma (1, 2),NoreenJ.Evans (3), Xiao-Xiao Ling (2, 4),Jin-HuiYang (2, 4),Fu-YuanWu (2, 4), Zhi-Dan Zhao (1)* and Yue-Heng Yang (2, 4)* (1) State Key Laboratory of Geological Processes and Mineral Resources, School of Earth Science and Resources, China University of Geosciences, Beijing 100083, China (2) State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China (3) School of Earth and Planetary Science, John de Laeter Centre, Curtin University, Perth, WA, Australia (4) Institutions of Earth Science, Chinese Academy of Sciences, Beijing 100029, China * Corresponding authors. e-mails: [email protected] and [email protected] Titanite is a common accessory mineral that preferentially incorporates considerable amounts of U and light rare earth elements in its structure, making it a versatile mineral for in situ U-Pb dating and Sm-Nd isotopic measurement. Here, we present in situ U-Pb ages and Sm-Nd isotope measurement results for four well-known titanite reference materials (Khan, BLR-1, OLT1 and MKED1) and eight titanite crystals that could be considered potential reference material candidates (Ontario, YQ-82, T3, T4, TLS-36, NW-IOA, Pakistan and C253), with ages ranging from ~ 20 Ma to ~ 1840 Ma. Results indicate that BLR-1, OLT1, Ontario, MKED1 and T3 titanite have relatively homogeneous Sm-Nd isotopes and low common Pb and thus can serve as primary reference materials for U-Pb and Sm-Nd microanalysis. -

List of Abbreviations
List of Abbreviations Ab albite Cbz chabazite Fa fayalite Acm acmite Cc chalcocite Fac ferroactinolite Act actinolite Ccl chrysocolla Fcp ferrocarpholite Adr andradite Ccn cancrinite Fed ferroedenite Agt aegirine-augite Ccp chalcopyrite Flt fluorite Ak akermanite Cel celadonite Fo forsterite Alm almandine Cen clinoenstatite Fpa ferropargasite Aln allanite Cfs clinoferrosilite Fs ferrosilite ( ortho) Als aluminosilicate Chl chlorite Fst fassite Am amphibole Chn chondrodite Fts ferrotscher- An anorthite Chr chromite makite And andalusite Chu clinohumite Gbs gibbsite Anh anhydrite Cld chloritoid Ged gedrite Ank ankerite Cls celestite Gh gehlenite Anl analcite Cp carpholite Gln glaucophane Ann annite Cpx Ca clinopyroxene Glt glauconite Ant anatase Crd cordierite Gn galena Ap apatite ern carnegieite Gp gypsum Apo apophyllite Crn corundum Gr graphite Apy arsenopyrite Crs cristroballite Grs grossular Arf arfvedsonite Cs coesite Grt garnet Arg aragonite Cst cassiterite Gru grunerite Atg antigorite Ctl chrysotile Gt goethite Ath anthophyllite Cum cummingtonite Hbl hornblende Aug augite Cv covellite He hercynite Ax axinite Czo clinozoisite Hd hedenbergite Bhm boehmite Dg diginite Hem hematite Bn bornite Di diopside Hl halite Brc brucite Dia diamond Hs hastingsite Brk brookite Dol dolomite Hu humite Brl beryl Drv dravite Hul heulandite Brt barite Dsp diaspore Hyn haiiyne Bst bustamite Eck eckermannite Ill illite Bt biotite Ed edenite Ilm ilmenite Cal calcite Elb elbaite Jd jadeite Cam Ca clinoamphi- En enstatite ( ortho) Jh johannsenite bole Ep epidote -

Band Gaps of Brookite, Rutile and Anatase
Optical Analysis of Titania: Band Gaps of Brookite, Rutile and Anatase Ryan Lance Advisor: Dr. Janet Tate A thesis presented in the partial fulfillment of the requirements for the degree of Bachelors of Physics Department of Physics Oregon State University May 5, 2018 Contents 1 Introduction 2 2 Optical Phenomena of Thin Films 3 2.1 The Index of Refraction . 3 2.2 Absorption . 4 2.3 The Band Gap . 5 3 Methods 6 3.1 The Grating Spectrometer . 6 3.2 SCOUT for Optical Modeling . 9 4 Results and Discussion 12 4.1 Band Gap Dependence on Thickness . 14 5 Conclusion 15 6 Appendix 16 6.1 Grating spectrometer settings . 16 6.2 Filtering 2nd Order Light . 16 6.3 Band gap of the substrate . 17 7 Using SCOUT 17 7.1 User configurations . 17 7.2 The Layer Stack . 18 7.3 Materials . 18 8 Acknowledgments 18 1 List of Figures 1 Indirect and direct band gaps . 5 2 The grating spectrometer. 7 3 TiO2 Raw Film Spectra . 7 4 Transmission, reflection, and corrected transmission spectra. 8 5 High-energy region of raw spectra . 9 6 Screenshot of the SCOUT interface . 10 7 Refractive index model constructed in SCOUT. 11 8 Density of states in the OJL band gap model. 11 9 High-fraction brookite film on SiO2 ....................... 12 10 High-fraction anatase film on SiO2 ....................... 13 11 High-fraction rutile film on SiO2 ........................ 13 12 Gap energy vs. Thickness for many polyphase TiO2 films. The phase plots (Rutile, Brookite, Anatase) show how much of each phase is present in each film.