SUPPLEMENTARY APPENDIX Multi-Center Randomized Open Label Phase II Trial on Three Rituximab Dosing Schemes in Immune Thrombocytopenia Pa - Tien
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regio Het Gooi Stads Taxi Prijzen: Hilversum: Hilversum – Hilversum 9
Regio Het Gooi Stads Taxi prijzen: Hilversum: Hilversum – Hilversum 9,50, Hilversum – Bussum 14,50, Hilversum – Naarden 19,50, Hilversum – Laren 14,50, Hilversum – Blaricum 19,50, Hilversum – Huizen 19,50, Hilversum – Eemnes 19,50, Hilversum – Baarn 14,50, Hilversum – Kortenhoef 14,50, Hilversum – s’Graveland 14,50, Hilversum – Nederhorst de berg 19,50, Hilversum – Ankeveen 14,50, Hilversum – Weesp 19,50, Hilversum – Muiden 19,50, Hilversum – Nigtevecht 19,50, Hilversum – Loenen a/d vecht 19,50, Hilversum – Vreeland 19,50, Hilversum – Loosdrecht 14,50 Bussum: Bussum – Bussum 9,50, Bussum – Hilversum 14,50, Bussum – Naarden 14,50, Bussum – Laren 14,50, Bussum – Blaricum 14,50, Bussum – Huizen 14,50, Bussum – Eemnes 19,50, Bussum – Baarn 19,50, Bussum – Kortenhoef 14,50, Bussum – s’Graveland 14,50, Bussum – Nederhorst den berg 19,50, Bussum – Ankeveen 14,50, Bussum – Weesp 19,50, Bussum – Muiden 19,50, Bussum – Nigtevecht 19,50, Bussum – Loenen a/d vecht 19,50, Bussum – Vreeland 19,50, Bussum – Loosdrecht 19,50 Naarden: Naarden – Naarden 9,50, Naarden – Hilversum 19,50, Naarden – Bussum 14,50, Naarden – Laren 14,50, Naarden – Blaricum 14,50, Naarden – Huizen 14,50, Naarden – Eemnes 19,50, Naarden – Baarn 19,50, Naarden – Kortenhoef 19,50, Naarden – s’Graveland 19,50, Naarden – Nederhorst den berg 19,50, Naarden – Ankeveen 19,50, Naarden – Weesp 14,50, Naarden – Muiden 14,50, Naarden – Nigtevecht 19,50, Naarden – Loenen a/d vecht 19,50, Naarden – Vreeland 19,50, Naarden – Loosdrecht 19,50 Laren: Laren – Laren 9,50, Laren – Hilversum 14,50, -

Indeling Van Nederland in 40 COROP-Gebieden Gemeentelijke Indeling Van Nederland Op 1 Januari 2019
Indeling van Nederland in 40 COROP-gebieden Gemeentelijke indeling van Nederland op 1 januari 2019 Legenda COROP-grens Het Hogeland Schiermonnikoog Gemeentegrens Ameland Woonkern Terschelling Het Hogeland 02 Noardeast-Fryslân Loppersum Appingedam Delfzijl Dantumadiel 03 Achtkarspelen Vlieland Waadhoeke 04 Westerkwartier GRONINGEN Midden-Groningen Oldambt Tytsjerksteradiel Harlingen LEEUWARDEN Smallingerland Veendam Westerwolde Noordenveld Tynaarlo Pekela Texel Opsterland Súdwest-Fryslân 01 06 Assen Aa en Hunze Stadskanaal Ooststellingwerf 05 07 Heerenveen Den Helder Borger-Odoorn De Fryske Marren Weststellingwerf Midden-Drenthe Hollands Westerveld Kroon Schagen 08 18 Steenwijkerland EMMEN 09 Coevorden Hoogeveen Medemblik Enkhuizen Opmeer Noordoostpolder Langedijk Stede Broec Meppel Heerhugowaard Bergen Drechterland Urk De Wolden Hoorn Koggenland 19 Staphorst Heiloo ALKMAAR Zwartewaterland Hardenberg Castricum Beemster Kampen 10 Edam- Volendam Uitgeest 40 ZWOLLE Ommen Heemskerk Dalfsen Wormerland Purmerend Dronten Beverwijk Lelystad 22 Hattem ZAANSTAD Twenterand 20 Oostzaan Waterland Oldebroek Velsen Landsmeer Tubbergen Bloemendaal Elburg Heerde Dinkelland Raalte 21 HAARLEM AMSTERDAM Zandvoort ALMERE Hellendoorn Almelo Heemstede Zeewolde Wierden 23 Diemen Harderwijk Nunspeet Olst- Wijhe 11 Losser Epe Borne HAARLEMMERMEER Gooise Oldenzaal Weesp Hillegom Meren Rijssen-Holten Ouder- Amstel Huizen Ermelo Amstelveen Blaricum Noordwijk Deventer 12 Hengelo Lisse Aalsmeer 24 Eemnes Laren Putten 25 Uithoorn Wijdemeren Bunschoten Hof van Voorst Teylingen -

Overzicht Drie Scenario's Middellange Termijn Perspectief Gooi En
BIJLAGE: Overzicht drie scenario’s middellange termijn perspectief Gooi en Vechtstreek A) Gooi-Noord en Gooi-Zuid In dit scenario fuseren de gemeenten Bussum, Huizen, Muiden en Naarden tot één gemeente met als werknaam ‘Gooi-Noord’ (+/- 100.000 inwoners) en de gemeenten Blaricum, Eemnes, Hilversum en Laren fuseren met elkaar tot een gemeente met als werknaam ‘Gooi-Zuid’ (+/- 115.000 inwoners). Daarnaast kunnen de gemeenten Stichtse Vecht, Weesp en Wijdemeren hun intensieve samenwerking verder uitbouwen. De Bloemendalerpolder wordt in zijn geheel aan Weesp toegevoegd. Grootschalige herindeling is in dit scenario noodzakelijk, omdat voor Muiden op korte termijn een herindeling nodig is. De vorming van alleen Gooi-Noord zou echter betekenen dat de positie van Hilversum als centrumgemeente onder druk komt te staan en bovendien zijn dan de verhoudingen van de gemeenten ten opzichte van de BEL-gemeenten zodanig scheef dat er geen sprake meer kan zijn van evenwichtige regionale verhoudingen als niet gelijktijdig ook Gooi-Zuid wordt gevormd. Er is ook geen sprake van restproblematiek, want er is geen gemeente die alleen overblijft. Kaart 1: Scenario Gooi-Noord en Gooi-Zuid B) Drie stromen In dit scenario fuseren Bussum, Hilversum, Muiden en Naarden met elkaar tot een gemeente van ongeveer 140.000 inwoners. Huizen en de BEL-gemeenten kunnen daarnaast komen tot een intensievere samenwerking of fusie voor zover dat nodig is om voldoende bestuurskracht te genereren. Aan de andere kant van de regio kunnen Stichtse Vecht, Weesp en Wijdemeren hun intensieve samenwerking vorm geven. De Bloemendalerpolder wordt in zijn geheel aan Weesp toegevoegd. De herindeling blijft in dit scenario beperkt tot de gemeenten die hebben uitgesproken een herindeling wenselijk te vinden. -

Betaalgedrag Individuele Gemeentes
Bijlage: Betaalgedrag individuele gemeentes Gemeente naam % tijdig Gemeente op basis van gemeentelijke betaald indeling 1-1-2021 Aa en Hunze 97% Aa en Hunze Aalsmeer 73% Aalsmeer Aalten 82% Aalten Achtkarspelen 81% Achtkarspelen Alblasserdam 82% Alblasserdam Albrandswaard 97% Albrandswaard Alkmaar 87% Alkmaar Almelo 89% Almelo Almere 73% Almere Alphen aan den Rijn 86% Alphen aan den Rijn Alphen-Chaam 90% Alphen-Chaam Werkendam 82% Altena Ameland 100% Ameland Amersfoort 94% Amersfoort Amstelveen 92% Amstelveen Amsterdam 79% Amsterdam Apeldoorn 78% Apeldoorn Arnhem 69% Arnhem Assen 91% Assen Asten 100% Asten Baarle-Nassau 99% Baarle-Nassau Baarn 99% Baarn Barendrecht 100% Barendrecht Barneveld 88% Barneveld Beek 93% Beek Schinnen 62% Beekdaelen Beemster 72% Beemster Beesel 92% Beesel Groesbeek 95% Berg en Dal Bergeijk 97% Bergeijk Bergen 99% Bergen Bergen (L) 97% Bergen (L) Bergen op Zoom 84% Bergen op Zoom Berkelland 96% Berkelland Bernheze 98% Bernheze Best 96% Best Beuningen 86% Beuningen Beverwijk 92% Beverwijk Bladel 96% Bladel Blaricum 78% Blaricum Bloemendaal 96% Bloemendaal 2 Gemeente naam % tijdig Gemeente op basis van gemeentelijke betaald indeling 1-1-2021 Bodegraven-Reeuwijk 97% Bodegraven-Reeuwijk Boekel 86% Boekel Borger-Odoorn 86% Borger-Odoorn Borne 100% Borne Borsele 100% Borsele Boxmeer 89% Boxmeer Boxtel 100% Boxtel Breda 81% Breda Brielle 89% Brielle Bronckhorst 94% Bronckhorst Brummen 93% Brummen Brunssum 81% Brunssum Bunnik 99% Bunnik Bunschoten 98% Bunschoten Buren 90% Buren Capelle aan den IJssel 89% Capelle aan den -

Fotokrant 'Metropool in Transitie'
Metropool in Transitie Metropoolregio Amsterdam Oktober 2018 1 Aalsmeer Almere Amstelveen Amsterdam Beemster Beverwijk Blaricum Bloemendaal Diemen Edam-Volendam Gooise Meren Haarlem Haarlemmerliede-Spaarnwoude Haarlemmermeer Heemskerk Heemstede Hilversum Huizen Landsmeer Laren Lelystad Oostzaan Ouder-Amstel Purmerend Uitgeest Uithoorn Velsen Waterland Weesp Wijdemeren Wormerland Zaanstad Zandvoort Aalsmeer Almere Amstelveen Amsterdam Beemster Beverwijk Blaricum Bloemendaal Diemen Edam-Volendam Gooise Meren Haarlem Haarlemmerliede-Spaarnwoude Haarlemmermeer Heemskerk Heemstede Hilversum Huizen Landsmeer Laren Lelystad Oostzaan Ouder-Amstel Purmerend Uitgeest Uithoorn Velsen Waterland Weesp Wijdemeren Wormerland Zaanstad Zandvoort AalsmeerInhoudso Almere Amstelveen Amsterdam Beemsterpg Beverwijkave Blaricum Bloemendaal Diemen Edam-Volendam Gooise Meren Haarlem Haarlemmerliede-Spaarnwoude Haarlemmermeer Voorwoord Heemskerk Heemstede Hilversum Huizen Landsmeer Laren Lelystad Oostzaan Ouder-Amstel Purmerend Uitgeest Uithoorn Velsen Waterland Weesp Wijdemeren Wormerland Zaanstad Zandvoort Aalsmeer Almere Amstelveen1 Voorwoord Amsterdam Beemster Beverwijk Blaricum Bloemendaal Ontwikkelingen in de wereld gaan ongelooflijk snel: op Diemen Edam-Volendam Gooise Meren Haarlem Haarlemmerliede-Spaarnwoude Haarlemmermeer uiteenlopende terreinen volgen veranderingen elkaar in Heemskerk Heemstede Hilversum HuizenFemke Halsema, Landsmeer burgemeester Laren Lelystad van Amsterdam Oostzaan Ouder-Amstel hoog tempo op. Bijzondere aandacht vragen de transities -

Cofinimmo Acquires a Healthcare Real Estate Site in the Netherlands
PRESS RELEASE Brussels, embargo until 04.07.2019, 17:40 CET Cofinimmo acquires a healthcare real estate site in the Netherlands Cofinimmo acquired through a subsidiary the medical office building ‘Regionaal Medisch Centrum Tergooi’ in Weesp (NL), close to Amsterdam, for approximately 7 million EUR. Jean-Pierre Hanin, CEO of Cofinimmo: “This transaction is fully in line with our objective to grow in the Netherlands. Our Dutch healthcare portfolio now reaches approximately 250 million EUR. With the foundation Tergooi, we welcome a stable operator with a clear care vision.” 1 PRESS RELEASE Brussels, embargo until 04.07.2019, 17:40 CET The site The building ‘Regionaal Medisch Centrum Tergooi’ was constructed in 1991. It is located in Weesp, about 15 km from the city centre of Amsterdam. The municipality is in full expansion. Construction works of 2,800 new houses started there in 2017, and in June 2019 Weesp and Amsterdam were officially merged. The building offers an above-ground surface area of 2,900 m². Since April 2019, with the opening of its brand new ‘Regionaal Medisch Centrum’, the foundation Tergooi became the main tenant. It has the possibility to rent supplementary surfaces according to the expansion of its activities and thus to perpetuate its presence on site in the long term. The building’s location between the city centre and the new urbanisation ensures an excellent access by public transport. The Weesp train station is within a few minutes’ walking distance. Works at the seller’s expense in order to optimise sustainability (and enabling the building to obtain a grade A energy label) are actually ongoing and will be completed in July 2019: new led lighting and new heating installation. -
Factsheet Zon-PV Noord-Holland-Zuid PDF Document
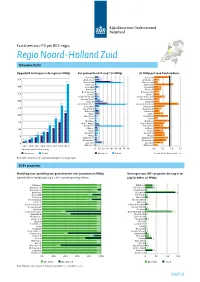
Factsheet zon-PV per RES-regio Regio Noord-Holland Zuid Totaaloverzicht Opgesteld vermogen in de regio (in MWp) Per gemeente eind 2019* (in MWp) (In MWp per 1000 huishoudens) 6 Aalsmeer 10 Aalsmeer 0,9 10 Amstelveen 12 Amstelveen 0,3 38 411 Amsterdam 64 Amsterdam 0,2 3 Beemster 4 Beemster 1,1 6 Beverwijk 8 Beverwijk 0,4 2 Blaricum 2 Blaricum 0,4 2 Bloemendaal 3 Bloemendaal 0,3 4 Diemen 4 Diemen 0,3 6 Edam-Volendam 8 Edam-Volendam 0,6 5 Gooise Meren 11 Gooise Meren 0,3 13 Haarlem 18 Haarlem 0,3 29 261 Haarlemmermeer 75 Haarlemmermeer 1,4 8 Heemskerk 10 Heemskerk 0,6 3 Heemstede 5 Heemstede 0,4 8 208 Hilversum 9 Hilversum 0,3 9 Huizen 9 Huizen 0,5 3 169 Landsmeer 4 Landsmeer 0,8 1 Laren 4 Laren 0,2 144 2 Oostzaan 5 Oostzaan 0,9 2 122 Ouder-Amstel 9 Ouder-Amstel 0,6 9 102 Purmerend 66 Purmerend 0,5 5 Uithoorn 7 Uithoorn 0,9 76 6 75 Velsen 8 Velsen 0,5 3 56 51 Waterland 7 Waterland 0,6 3 34 38 Weesp 5 Weesp 0,6 4 23 Wijdemeren 5 Wijdemeren 0,8 9 15 4 Wormerland 4 Wormerland 0,7 16 Zaanstad 18 Zaanstad 0,5 1 0,2 * Zandvoort 3 Zandvoort *(per einde van het kalenderjaar) , , , , Woningen Totaal Woningen Totaal Gemiddeld in Nederland: 0,9 Bron: CBS – Zonnestroom: opgesteld vermogen *voorlopige cijfers SDE+ projecten Verdeling naar opstelling van gerealiseerde sde+ projecten (in MWp) Vermogen van SDE+ projecten die nog in de Gemiddeld in Nederland: 63% SDE+ gerealiseerd op daken pijplijn zitten (in MWp) 33 Aalsmeer 100% Aalsmeer 33 34 Amstelveen 100% Amstelveen 34 6 Amsterdam 94% Amsterdam 7 135 Beemster 100% Beemster -

1. Regionale Samenwerkingsagenda 2019-2022 (Regio Gooi En
Van: Jaap-Jan Bakker [mailto:[email protected]] Verzonden: dinsdag 2 oktober 2018 9:56 Aan: '[email protected]' <[email protected]>; '[email protected]' <[email protected]>; '[email protected]' <[email protected]>; Jongerden, Magda <[email protected]>; Driessen, Constance <[email protected]>; 'bestuurssecretariaat Blaricum ([email protected])' <[email protected]>; '[email protected]' <[email protected]>; 'Bestuurssecretariaat Laren ([email protected])' <[email protected]>; '[email protected]' <[email protected]>; ''Gemeente Weesp' ([email protected])' <[email protected]>; '[email protected]' <[email protected]> CC: 'Paul van Ruitenbeek' <[email protected]>; '[email protected]' <[email protected]>; 'Debbie de Heus ' <[email protected]>; '[email protected]' <[email protected]>; 'griffie Gooise Meren' <[email protected]>; Veenstra, Jan <[email protected]>; Jaap-Jan Bakker <[email protected]>; 'Corrie Holtslag ' <[email protected]>; 'Martin van Engelshoven ' <[email protected]>; 'mw. A.H. de Graaf' <[email protected]>; '[email protected]' <[email protected]>; '[email protected]' <[email protected]>; ''[email protected]' ([email protected])' <[email protected]>; '[email protected]' <[email protected]>; '[email protected]' <[email protected]>; '[email protected]' <[email protected]>; '[email protected]' -

“Blaricum, Authentiek Dorp in 'T Gooi”
“BlarIcum, authentIek dorp In ‘t GooI” Strategische visie Blaricum 2030 waarin opgenomen structuurvisie In opdracht van de Gemeente Blaricum, vastgesteld in de gemeenteraadsvergadering van 26 januari 2010 “BlarIcum, authentIek dorp In ‘t GooI” Blaricum, authentiek dorp in ‘t Gooi colofon januari 2010 In opdracht van Gemeente Blaricum Projectleider: Jeroen Verburg Início Esmeralda van Tuinen, Sander Nieuwenhuis en Annemarij Swart Heemraadssingel 111 3022 CC Rotterdam 010-2210000 www.inicio.nl Foto’s: Início, Gemeente Blaricum, Gerard Grootveld Tekeningen visie: Luuk Poorthuis Opmaak en kaarten visie: Paul Stoute De originele versie van dit rapport bevat kleurenafbeeldingen. Início is niet verantwoordelijk voor kwaliteits- en informatieverlies bij kopiëren. 4 Inhoudsopgave Inhoudsopgave pag. 1. Inleiding 5 1.1 Blaricum, authentiek dorp in ‘t Gooi 5 1.2 Werkwijze 5 1.3 Leeswijzer 5 2. Juridisch kader structuurvisie Blaricum 7 2.1 Aanleiding 7 2.2 Doel 7 2.3 Wettelijk kader 7 2.4 Uitwerking 8 3. Identiteit 9 3.1 Historie 9 3.2 Lekkers dorps 9 3.3 Gezellig samen… 9 3.4 …maar ook individueel 9 3.5 Belangrijkste kernwaarden: groen, vrijheid, rust en authentiek 10 4. Analyse 11 4.1 Sociale kenmerken 11 ±± 4.2 Fysieke kenmerken 11 4.3 Mobiliteitskenmerken 12 4.4 Economische kenmerken 12 4.5 Organisatorische/bestuurlijke samenwerking 14 4.6 SWOT-overzicht 16 4.7 Samenvatting analyse 18 5. Visie 19 5 Blaricum, authentiek dorp in ‘t Gooi 6. Missie 21 6.1 Ontmoeten stimuleren 21 6.2 Fysieke verbindingen versterken 21 6.3 Recreatie verbeteren 22 6.4 Centrumgebieden meer kwaliteit geven 23 6.5 Handhaven dorpse karakter 23 6.6 Streven naar een evenwichtige bevolkingsopbouw 24 6.7 Randvoorwaarden: kleinschaligheid behouden en samenwerking regio versterken 24 7. -

Club Health Assessment MBR0087
Club Health Assessment for District 110AN through January 2021 Status Membership Reports Finance LCIF Current YTD YTD YTD YTD Member Avg. length Months Yrs. Since Months Donations Member Members Members Net Net Count 12 of service Since Last President Vice Since Last for current Club Club Charter Count Added Dropped Growth Growth% Months for dropped Last Officer Rotation President Activity Account Fiscal Number Name Date Ago members MMR *** Report Reported Report *** Balance Year **** Number of times If below If net loss If no When Number Notes the If no report on status quo 15 is greater report in 3 more than of officers thatin 12 months within last members than 20% months one year repeat do not haveappears in two years appears appears appears in appears in terms an active red Clubs less than two years old S,T,M,VP,MC 142238 Roma Amsterdam Global 07/22/2020 Active 49 49 0 49 100.00% 0 1 3 R N/A $162.57 SC Clubs more than two years old P,S,T,M,VP 30740 AALSMEER 05/20/1975 Active 28 0 0 0 0.00% 27 4 N/A $98.96 MC,SC P,S,T,M,VP 57413 AALSMEER OPHELIA 10/16/1995 Active 22 1 0 1 4.76% 22 3 N/A $74.22 MC,SC P,S,T,M,VP 40649 ABCOUDE BAAMBRUGGE 12/23/1981 Active 32 3 0 3 10.34% 31 0 N/A $102.49 MC,SC S,T,M,VP,MC 21347 ALKMAAR 10/15/1959 Active 11 0 1 -1 -8.33% 11 8 4 N/A $38.87 SC P,M,VP,MC,SC 47289 ALKMAAR PHOENIX 04/30/1987 Active 15 1 0 1 7.14% 13 6 3 N/R $53.01 T,M,VP,MC,SC 48550 ALMERE HOST 05/02/1988 Active 16 0 0 0 0.00% 20 5 N/R $102.49 S,M,VP,MC,SC 105327 ALMERE VERITAS 04/21/2009 Active 14 0 0 0 0.00% 14 3 N/A $49.48 M,VP,MC,SC 21349 -

Blaricum, Authentiek Dorp in Het Gooi Coalitieakkoord 2018-2022
BLARICUM, AUTHENTIEK DORP IN HET GOOI COALITIEAKKOORD 2018-2022 HART VOOR BLARICUM DE BLARICUMSE PARTIJ CDA 23 april 2018 1 Inhoud Voorwoord ............................................................................................................................................... 3 1. Blaricum behoudt bestuurskracht en blijft zelfstandig .................................................................... 4 2. Blaricum behoudt zijn kernwaarden en kroonjuwelen .................................................................... 5 3. Wij communiceren goed en geven ruimte aan (burger)participatie ............................................... 5 4. Extra aandacht voor de veiligheid van onze inwoners .................................................................... 6 5. Extra aandacht voor onderhoud van groen, wegen, water ............................................................. 6 6. We geven prioriteit aan verkeersmaatregelen (veiligheid, knelpunten, busverbindingen) ............ 7 7. We koesteren de Blaricumse evenementen, cultuur, recreatie en sportvoorzieningen .................. 7 8. We brengen focus aan binnen het sociaal domein ......................................................................... 8 9. Milieu: duurzaamheidsbeleid concretiseren en uitvoeren ............................................................... 9 10. We voeren de woonvisie uit en voltooien de Blaricummermeent ................................................. 10 11. Onze financiën zijn en blijven op orde, zonder lastenverzwaring ................................................. -

Airbnb in Noord-Holland
Airbnb in Noord-Holland Door: Maarten Sukel [email protected] december 2018 Onderzoek uitgevoerd door BeFormation in opdracht van provincie Noord-Holland 1. INHOUDSOPGAVE 2. Inleiding ....................................................................................................................................................................... 3 Definities .................................................................................................................................................................................. 3 Methode................................................................................................................................................................................... 4 Onderzoeksgebied.................................................................................................................................................................... 5 Onderzoeksperiode .................................................................................................................................................................. 5 3. Aantallen ..................................................................................................................................................................... 6 Advertenties ............................................................................................................................................................................. 6 Aanbieders ............................................................................................................................................................................