Characterization and Computational Simulation of Human Syx, a Rhogef Implicated in Glioblastoma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Supp Table 6.Pdf
Supplementary Table 6. Processes associated to the 2037 SCL candidate target genes ID Symbol Entrez Gene Name Process NM_178114 AMIGO2 adhesion molecule with Ig-like domain 2 adhesion NM_033474 ARVCF armadillo repeat gene deletes in velocardiofacial syndrome adhesion NM_027060 BTBD9 BTB (POZ) domain containing 9 adhesion NM_001039149 CD226 CD226 molecule adhesion NM_010581 CD47 CD47 molecule adhesion NM_023370 CDH23 cadherin-like 23 adhesion NM_207298 CERCAM cerebral endothelial cell adhesion molecule adhesion NM_021719 CLDN15 claudin 15 adhesion NM_009902 CLDN3 claudin 3 adhesion NM_008779 CNTN3 contactin 3 (plasmacytoma associated) adhesion NM_015734 COL5A1 collagen, type V, alpha 1 adhesion NM_007803 CTTN cortactin adhesion NM_009142 CX3CL1 chemokine (C-X3-C motif) ligand 1 adhesion NM_031174 DSCAM Down syndrome cell adhesion molecule adhesion NM_145158 EMILIN2 elastin microfibril interfacer 2 adhesion NM_001081286 FAT1 FAT tumor suppressor homolog 1 (Drosophila) adhesion NM_001080814 FAT3 FAT tumor suppressor homolog 3 (Drosophila) adhesion NM_153795 FERMT3 fermitin family homolog 3 (Drosophila) adhesion NM_010494 ICAM2 intercellular adhesion molecule 2 adhesion NM_023892 ICAM4 (includes EG:3386) intercellular adhesion molecule 4 (Landsteiner-Wiener blood group)adhesion NM_001001979 MEGF10 multiple EGF-like-domains 10 adhesion NM_172522 MEGF11 multiple EGF-like-domains 11 adhesion NM_010739 MUC13 mucin 13, cell surface associated adhesion NM_013610 NINJ1 ninjurin 1 adhesion NM_016718 NINJ2 ninjurin 2 adhesion NM_172932 NLGN3 neuroligin -

Identification of Transcriptional Mechanisms Downstream of Nf1 Gene Defeciency in Malignant Peripheral Nerve Sheath Tumors Daochun Sun Wayne State University
Wayne State University DigitalCommons@WayneState Wayne State University Dissertations 1-1-2012 Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors Daochun Sun Wayne State University, Follow this and additional works at: http://digitalcommons.wayne.edu/oa_dissertations Recommended Citation Sun, Daochun, "Identification of transcriptional mechanisms downstream of nf1 gene defeciency in malignant peripheral nerve sheath tumors" (2012). Wayne State University Dissertations. Paper 558. This Open Access Dissertation is brought to you for free and open access by DigitalCommons@WayneState. It has been accepted for inclusion in Wayne State University Dissertations by an authorized administrator of DigitalCommons@WayneState. IDENTIFICATION OF TRANSCRIPTIONAL MECHANISMS DOWNSTREAM OF NF1 GENE DEFECIENCY IN MALIGNANT PERIPHERAL NERVE SHEATH TUMORS by DAOCHUN SUN DISSERTATION Submitted to the Graduate School of Wayne State University, Detroit, Michigan in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY 2012 MAJOR: MOLECULAR BIOLOGY AND GENETICS Approved by: _______________________________________ Advisor Date _______________________________________ _______________________________________ _______________________________________ © COPYRIGHT BY DAOCHUN SUN 2012 All Rights Reserved DEDICATION This work is dedicated to my parents and my wife Ze Zheng for their continuous support and understanding during the years of my education. I could not achieve my goal without them. ii ACKNOWLEDGMENTS I would like to express tremendous appreciation to my mentor, Dr. Michael Tainsky. His guidance and encouragement throughout this project made this dissertation come true. I would also like to thank my committee members, Dr. Raymond Mattingly and Dr. John Reiners Jr. for their sustained attention to this project during the monthly NF1 group meetings and committee meetings, Dr. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Rho Guanine Nucleotide Exchange Factors: Regulators of Rho Gtpase Activity in Development and Disease
Oncogene (2014) 33, 4021–4035 & 2014 Macmillan Publishers Limited All rights reserved 0950-9232/14 www.nature.com/onc REVIEW Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease DR Cook1, KL Rossman2,3 and CJ Der1,2,3 The aberrant activity of Ras homologous (Rho) family small GTPases (20 human members) has been implicated in cancer and other human diseases. However, in contrast to the direct mutational activation of Ras found in cancer and developmental disorders, Rho GTPases are activated most commonly in disease by indirect mechanisms. One prevalent mechanism involves aberrant Rho activation via the deregulated expression and/or activity of Rho family guanine nucleotide exchange factors (RhoGEFs). RhoGEFs promote formation of the active GTP-bound state of Rho GTPases. The largest family of RhoGEFs is comprised of the Dbl family RhoGEFs with 70 human members. The multitude of RhoGEFs that activate a single Rho GTPase reflects the very specific role of each RhoGEF in controlling distinct signaling mechanisms involved in Rho activation. In this review, we summarize the role of Dbl RhoGEFs in development and disease, with a focus on Ect2 (epithelial cell transforming squence 2), Tiam1 (T-cell lymphoma invasion and metastasis 1), Vav and P-Rex1/2 (PtdIns(3,4,5)P3 (phosphatidylinositol (3,4,5)-triphosphate)-dependent Rac exchanger). Oncogene (2014) 33, 4021–4035; doi:10.1038/onc.2013.362; published online 16 September 2013 Keywords: Rac1; RhoA; Cdc42; guanine nucleotide exchange factors; cancer; -

A Novel FGD1 Mutation in a Family with Aarskog–Scott Syndrome And
COLD SPRING HARBOR Molecular Case Studies | RESEARCH REPORT AnovelFGD1 mutation in a family with Aarskog–Scott syndrome and predominant features of congenital joint contractures Laurie Beth Griffin,1,2 Frances A. Farley,3 Anthony Antonellis,1,4,5 and Catherine E. Keegan4,6 1Program in Cellular and Molecular Biology, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA; 2Medical Scientist Training Program, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA; 3Department of Orthopaedic Surgery, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA; 4Department of Human Genetics, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA; 5Department of Neurology, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA; 6Department of Pediatrics, University of Michigan Medical School, Ann Arbor, Michigan 48109, USA Abstract Mutations in FGD1 cause Aarskog–Scott syndrome (AAS), an X-linked condition characterized by abnormal facial, skeletal, and genital development due to abnormal embryonic morphogenesis and skeletal formation. Here we report a novel FGD1 mutation in a family with atypical features of AAS, specifically bilateral upper and lower limb Corresponding author: congenital joint contractures and cardiac abnormalities. The male proband and his affected [email protected] maternal uncle are hemizygous for the novel FGD1 mutation p.Arg921X. This variant is the most carboxy-terminal FGD1 mutation identified in a family with AAS and is predicted to © 2016 Griffin et al. This article is truncate the FGD1 protein at the second to last amino acid of the carboxy-terminal distributed under the terms of pleckstrin homology (PH) domain. Our study emphasizes the importance of the 3′ peptide the Creative Commons sequence in the structure and/or function of the FGD1 protein and further demonstrates Attribution-NonCommercial the need to screen patients with X-linked congenital joint contractures for FGD1 mutations. -

Trio's Rho-Specific GEF Domain Is the Missing G Q Effector in C. Elegans
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press Trio’s Rho-specific GEF domain is ␣ the missing G q effector in C. elegans Stacey L. Williams,1 Susanne Lutz,2 Nicole K. Charlie,1 Christiane Vettel,2 Michael Ailion,3 Cassandra Coco,4 John J.G. Tesmer,4 Erik M. Jorgensen,3 Thomas Wieland,2 and Kenneth G. Miller1,5 1Program in Molecular, Cell, and Developmental Biology, Oklahoma Medical Research Foundation, Oklahoma City, Oklahoma 73104, USA; 2Institute of Experimental and Clinical Pharmacology, Medical Faculty Mannheim, University of Heidelberg, D-68169 Mannheim, Germany; 3Department of Biology, Howard Hughes Medical Institute, University of Utah, Salt Lake City, Utah 84112, USA; 4Department of Pharmacology, Life Sciences Institute, University of Michigan, Ann Arbor, Michigan 48109, USA ␣ The G q pathway is essential for animal life and is a central pathway for driving locomotion, egg laying, and growth in Caenorhabditis elegans, where it exerts its effects through EGL-8 (phospholipase C [PLC]) and at least one other effector. To find the missing effector, we performed forward genetic screens to suppress the ␣ slow growth and hyperactive behaviors of mutants with an overactive G q pathway. Four suppressor mutations disrupted the Rho-specific guanine-nucleotide exchange factor (GEF) domain of UNC-73 (Trio). The mutations produce defects in neuronal function, but not neuronal development, that cause sluggish locomotion similar to animals lacking EGL-8 (PLC). Strains containing null mutations in both EGL-8 (PLC) and UNC-73 (Trio RhoGEF) have strong synthetic phenotypes that phenocopy the arrested growth and ␣ near-complete paralysis of G q-null mutants. -

Guanine Nucleotide Exchange Factors for Rho Gtpases: Turning on the Switch
Downloaded from genesdev.cshlp.org on September 27, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Guanine nucleotide exchange factors for Rho GTPases: turning on the switch Anja Schmidt1,3 and Alan Hall1,2 1MRC Laboratory for Molecular Cell Biology, Cancer Research UK Oncogene and Signal Transduction Group, and 2Department of Biochemistry and Molecular Biology, University College London, London WC1E 6BT, UK Rho GTPases control many aspects of cell behavior Structural features through the regulation of multiple signal transduction The first mammalian GEF, Dbl, isolated in 1985 as an pathways (Van Aelst and D’Souza-Schorey 1997; Hall oncogene in an NIH 3T3 focus formation assay using 1998). Rho, Rac, and Cdc42were first recognized in the DNA from a human diffuse B-cell lymphoma (Eva and early 1990s for their unique ability to induce specific ∼ filamentous actin structures in fibroblasts; stress fibers, Aaronson 1985), was found to contain a region of 180 lamellipodia/membrane ruffles, and filopodia, respec- amino acids that showed significant sequence similarity tively (Hall 1998). Over the intervening years, evidence to CDC24, a protein identified genetically as an up- has accumulated to show that in all eukaryotic cells, stream activator of CDC42in yeast (Bender and Pringle Rho GTPases are involved in most, if not all, actin-de- 1989; Ron et al. 1991). Dbl was subsequently shown to pendent processes such as those involved in migration, catalyze nucleotide exchange on human Cdc42in vitro adhesion, morphogenesis, axon guidance, and phagocy- (Hart et al. 1991), and a conserved domain in Dbl and tosis (Kaibuchi et al. 1999; Chimini and Chavrier 2000; CDC24, now known as the DH (Dbl homology) domain, Luo 2000). -

A Systems Immunology Approach Identifies the Collective Impact of Five Mirs in Th2 Inflammation Supplementary Information
A systems immunology approach identifies the collective impact of five miRs in Th2 inflammation Supplementary information Ayşe Kılıç, Marc Santolini, Taiji Nakano, Matthias Schiller, Mizue Teranishi,Pascal Gellert, Yuliya Ponomareva, Thomas Braun, Shizuka Uchida, Scott T. Weiss, Amitabh Sharma and Harald Renz Corresponding Author: Harald Renz, MD Institute of Laboratory Medicine Philipps University Marburg 35043 Marburg, Germany Phone +49 6421 586 6235 [email protected] 1 Kılıç A et. al. 2018 A B C D PBS OVA chronic PAS PAS (cm H2O*s/ml (cm Raw Sirius Red Sirius Supplementary figure 1: Characteristic phenotype of the acute and chronic allergic airway inflammatory response in the OVA-induced mouse model. (a) Total and differential cell counts determined in BAL and cytospins show a dominant influx of eosinophils. (b) Serum titers of OVA-specific immunoglobulins are elevated. (c) Representative measurement of airway reactivity to increasing methacholine (Mch) responsiveness measured by head-out body plethysmography. (d) Representative lung section staining depicting airway inflammation and mucus production (PAS, original magnification x 10) and airway remodeling (SiriusRed; original magnification x 20). Data are presented as mean ± SEM and are representative for at least 3 independent experiments with n=8-10 animals per group. Statistical analyses have been performed with one-way ANOVA and Tukey’s post-test and show *p<0.05, **p<0.01 and ***p<0.001. 2 Kılıç A et. al. 2018 lymphocytes A lung activated CD4+-cells CD4+-T-cell-subpopulations SSC FSC ST2 live CD69 CD4 CXCR3 B spleen Naive CD4+- T cells DAPI FSC single cells SSC CD62L CD4 CD44 FSC FSC-W Supplementary figure 2. -

NIH Public Access Author Manuscript Nat Rev Cancer
NIH Public Access Author Manuscript Nat Rev Cancer. Author manuscript; available in PMC 2011 June 27. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Nat Rev Cancer. 2010 December ; 10(12): 842±857. doi:10.1038/nrc2960. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Dominico Vigil*, Jacqueline Cherfils‡, Kent L. Rossman*, and Channing J. Der* * University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, Department of Pharmacology, Chapel Hill, NC 27599 ‡ Laboratoire d’Enzymologie et Biochimie Structurales, CNRS, avenue de la Terrasse, 91198 Gif sur Yvette Cedex, France Abstract There is now considerable and increasing evidence for a causal role of aberrant activity of the Ras superfamily of small GTPases in human cancers. These GTPases act as GDP-GTP-regulated binary switches that control many fundamental cellular processes. A common mechanism of GTPase deregulation in cancer is the deregulated expression and/or activity of their regulatory proteins, guanine nucleotide exchange factors (GEFs) that promote formation of the active GTP- bound state and GTPase activating proteins (GAPs) that return the GTPase to its GDP-bound inactive state. We assess the association of GEFs and GAPs with cancer and their druggability for cancer therapeutics. Ras proteins (H-, N- and K-Ras) are the founding members of a large superfamily of monomeric small GTPases (20–25 kDa) that regulate diverse cellular processes that include cell cycle progression, cell survival, actin cytoskeletal organization, cell polarity and movement, and vesicular and nuclear transport1, 2. The Ras superfamily (>150 members in humans) is divided into five main families based on sequence identity and function: Ras, Rho, Rab, Arf, and Ran (BOX 1). -

Son of Sevenless Directly Links the Robo Receptor to Rac Activation to Control Axon Repulsion at the Midline
Neuron 52, 595–607, November 22, 2006 ª2006 Elsevier Inc. DOI 10.1016/j.neuron.2006.09.039 Son of Sevenless Directly Links the Robo Receptor to Rac Activation to Control Axon Repulsion at the Midline Long Yang1 and Greg J. Bashaw1,* type III repeats, a single transmembrane domain, and 1 Department of Neuroscience a long cytoplasmic tail that contains four blocks of con- University of Pennsylvania School of Medicine served cytoplasmic (CC) sequences (CC0, CC1, CC2, 421 Curie Boulevard CC3) (Bashaw et al., 2000; Kidd et al., 1998). Robo is re- Philadelphia, Pennsylvania 19104 quired to prevent axons from inappropriately crossing the CNS midline in both invertebrates and vertebrates, and it has also been implicated in controlling cell migra- Summary tion in other cell types (Kidd et al., 1998; Kramer et al., 2001; Long et al., 2004). In Drosophila, mutations in Son of sevenless (Sos) is a dual specificity guanine robo and its midline-expressed ligand slit result in too nucleotide exchange factor (GEF) that regulates both many axons crossing and staying at the midline (Kidd Ras and Rho family GTPases and thus is uniquely et al., 1999; Seeger et al., 1993). Several proteins that poised to integrate signals that affect both gene ex- regulate the actin cytoskeleton, including the cytoplas- pression and cytoskeletal reorganization. Here, using mic tyrosine kinase Abelson (Abl) and its substrate En- genetics, biochemistry, and cell biology, we demon- abled (Ena), contribute to the Robo signaling pathway strate that Sos is recruited to the plasma membrane, in Drosophila and C. elegans (Bashaw et al., 2000; where it forms a ternary complex with the Roundabout Hsouna et al., 2003; Wills et al., 2002; Yu et al., 2002). -
Downloaded from the Mouse Lysosome Gene Database, Mlgdb
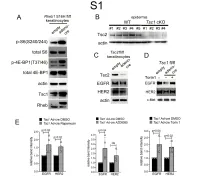
1 Supplemental Figure Legends 2 3 Supplemental Figure S1: Epidermal-specific mTORC1 gain-of-function models show 4 increased mTORC1 activation and down-regulate EGFR and HER2 protein expression in a 5 mTORC1-sensitive manner. (A) Immunoblotting of Rheb1 S16H flox/flox keratinocyte cultures 6 infected with empty or adenoviral cre recombinase for markers of mTORC1 (p-S6, p-4E-BP1) 7 activity. (B) Tsc1 cKO epidermal lysates also show decreased expression of TSC2 by 8 immunoblotting of the same experiment as in Figure 2A. (C) Immunoblotting of Tsc2 flox/flox 9 keratinocyte cultures infected with empty or adenoviral cre recombinase showing decreased EGFR 10 and HER2 protein expression. (D) Expression of EGFR and HER2 was decreased in Tsc1 cre 11 keratinocytes compared to empty controls, and up-regulated in response to Torin1 (1µM, 24 hrs), 12 by immunoblot analyses. Immunoblots are contemporaneous and parallel from the same biological 13 replicate and represent the same experiment as depicted in Figure 7B. (E) Densitometry 14 quantification of representative immunoblot experiments shown in Figures 2E and S1D (r≥3; error 15 bars represent STDEV; p-values by Student’s T-test). 16 17 18 19 20 21 22 23 Supplemental Figure S2: EGFR and HER2 transcription are unchanged with epidermal/ 24 keratinocyte Tsc1 or Rptor loss. Egfr and Her2 mRNA levels in (A) Tsc1 cKO epidermal lysates, 25 (B) Tsc1 cKO keratinocyte lysates and(C) Tsc1 cre keratinocyte lysates are minimally altered 26 compared to their respective controls. (r≥3; error bars represent STDEV; p-values by Student’s T- 27 test).