Guanine Nucleotide Exchange Factors for Rho Gtpases: Turning on the Switch
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
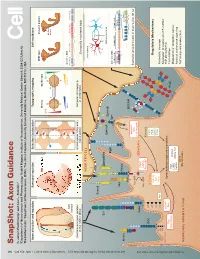
Snapshot: Axon Guidance Pasterkamp R
494 1 Cell Cell ??? SnapShot: Axon Guidance 153 SnapShot: XXXXXXXXXXXXXXXXXXXXXXXXXX 1 2 , ??MONTH?? ??DATE??, 200? ©200? Elsevier Inc. 200?©200? ElsevierInc. , ??MONTH?? ??DATE??, DOI R. Jeroen Pasterkamp and Alex L. Kolodkin , April11, 2013©2013Elsevier Inc. DOI http://dx.doi.org/10.1016/j.cell.2013.03.031 AUTHOR XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX 1 AFFILIATIONDepartment of XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Neuroscience and Pharmacology, Rudolf Magnus Institute of Neuroscience, University Medical Center Utrecht, 3584 CG Utrecht, The Netherlands; 2Department of Neuroscience, HHMI, The Johns Hopkins University School of Medicine, Baltimore, MD 21212, USA Axon attraction and repulsion Surround repulsion Selective fasciculation Topographic mapping Self-avoidance Wild-type Dscam1 mutant Sema3A A Retina SC/Tectum EphB EphrinB P D D Mutant T A neuron N P A V V Genomic DNA P EphA EphrinA Slit Exon 4 (12) Exon 6 (48) Exon 9 (33) Exon 17 (2) Netrin Commissural axon guidance Surround repulsion of peripheral Grasshopper CNS axon Retinotectal mapping at the CNS midline nerves in vertebrates fasciculation in vertebrates Drosophila mushroom body XXXXXXXXX NEURITE/CELL Isoneuronal Sema3 Slit Sema1/4-6 Heteroneuronal EphrinA EphrinB FasII Eph Genomic DNA Pcdh-α (14) Pcdh-β (22) Pcdh-γ (22) Variable Con Variable Con Nrp Plexin ** *** Netrin LAMELLIPODIA Con DSCAM ephexin Starburst amacrine cells in mammalian retina Ras-GTP Vav See online version for legend and references. α-chimaerin GEFs/GAPs Robo FARP Ras-GDP LARG RhoGEF Kinases DCC cc0 GTPases PKA cc1 FAK Regulatory Mechanisms See online versionfor??????. Cdc42 GSK3 cc2 Rac PI3K P1 Rho P2 cc3 Abl Proteolytic cleavage P3 Regulation of expression (TF, miRNA, FILOPODIA srGAP Cytoskeleton regulatory proteins multiple isoforms) Sos Trio Pcdh Cis inhibition DOCK180 PAK ROCK Modulation of receptors’ output LIMK Myosin-II Colin Forward and reverse signaling Actin Trafcking and endocytosis NEURONAL GROWTH CONE Microtubules SnapShot: Axon Guidance R. -

Paxillin Binding to the Cytoplasmic Domain of CD103 Promotes Cell Adhesion and Effector
Author Manuscript Published OnlineFirst on October 11, 2017; DOI: 10.1158/0008-5472.CAN-17-1487 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Paxillin binding to the cytoplasmic domain of CD103 promotes cell adhesion and effector functions for CD8+ resident memory T cells in tumors Ludiane Gauthier1, Stéphanie Corgnac1, Marie Boutet1, Gwendoline Gros1, Pierre Validire2, Georges Bismuth3 and Fathia Mami-Chouaib1 1 INSERM UMR 1186, Integrative Tumor Immunology and Genetic Oncology, Gustave Roussy, EPHE, Fac. de médecine - Univ. Paris-Sud, Université Paris-Saclay, 94805, Villejuif, France 2 Institut Mutualiste Montsouris, Service d’Anatomie pathologique, 75014 Paris, France. 3 INSERM U1016, CNRS UMR8104, Université Paris Descartes, Institut Cochin, 75014 Paris. S Corgnac, M Boutet and G Gros contributed equally to this work. M Boutet current address: Department of Microbiology and Immunology Albert Einstein College of Medecine, NY 10461 USA. Corresponding author: Fathia Mami-Chouaib, INSERM UMR 1186, Gustave Roussy. 39, rue Camille Desmoulins, F-94805 Villejuif. Phone: +33 1 42 11 49 65, Fax: +33 1 42 11 52 88, e-mail: [email protected] and [email protected] Running title: CD103 signaling in human TRM cells Key words: TRM cells, CD103 integrin, T-cell function and signaling, paxillin. Abbreviations: IS: immune synapse; LFA: leukocyte function-associated antigen; FI: fluorescence intensity; mAb: monoclonal antibody; phospho: phosphorylated; Pyk2: proline- rich tyrosine kinase-2; NSCLC: non-small-cell lung carcinoma; r: recombinant; sh-pxn: shorthairpin RNA-paxillin; TCR: T-cell receptor; TIL: tumor-infiltrating lymphocyte; TRM: tissue-resident memory T. -

Regulation of the Mammalian Target of Rapamycin Complex 2 (Mtorc2)
Regulation of the Mammalian Target Of Rapamycin Complex 2 (mTORC2) Inauguraldissertation Zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Klaus-Dieter Molle aus Heilbronn, Deutschland Basel, 2006 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakultät Auf Antrag von Prof. Michael N. Hall und Prof. Markus Affolter. Basel, den 21.11.2006 Prof. Hans-Peter Hauri Dekan Summary The growth controlling mammalian Target of Rapamycin (mTOR) is a conserved Ser/Thr kinase found in two structurally and functionally distinct complexes, mTORC1 and mTORC2. The tumor suppressor TSC1-TSC2 complex inhibits mTORC1 by acting on the small GTPase Rheb, but the role of TSC1-TSC2 and Rheb in the regulation of mTORC2 is unclear. Here we examined the role of TSC1-TSC2 in the regulation of mTORC2 in human embryonic kidney 293 cells. Induced knockdown of TSC1 and TSC2 (TSC1/2) stimulated mTORC2-dependent actin cytoskeleton organization and Paxillin phosphorylation. Furthermore, TSC1/2 siRNA increased mTORC2-dependent Ser473 phosphorylation of plasma membrane bound, myristoylated Akt/PKB. This suggests that loss of Akt/PKB Ser473 phosphorylation in TSC mutant cells, as reported previously, is due to inhibition of Akt/PKB localization rather than inhibition of mTORC2 activity. Amino acids and overexpression of Rheb failed to stimulate mTORC2 signaling. Thus, TSC1-TSC2 also inhibits mTORC2, but possibly independently of Rheb. Our results suggest that mTORC2 hyperactivation may contribute to the pathophysiology of diseases such as cancer and Tuberous Sclerosis Complex. i Acknowledgement During my PhD studies in the Biozentrum I received a lot of support from many people around me who I mention here to express my gratefulness. -

The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination
The Journal of Neuroscience, August 31, 2011 • 31(35):12579–12592 • 12579 Cellular/Molecular The Atypical Guanine-Nucleotide Exchange Factor, Dock7, Negatively Regulates Schwann Cell Differentiation and Myelination Junji Yamauchi,1,3,5 Yuki Miyamoto,1 Hajime Hamasaki,1,3 Atsushi Sanbe,1 Shinji Kusakawa,1 Akane Nakamura,2 Hideki Tsumura,2 Masahiro Maeda,4 Noriko Nemoto,6 Katsumasa Kawahara,5 Tomohiro Torii,1 and Akito Tanoue1 1Department of Pharmacology and 2Laboratory Animal Resource Facility, National Research Institute for Child Health and Development, Setagaya, Tokyo 157-8535, Japan, 3Department of Biological Sciences, Tokyo Institute of Technology, Midori, Yokohama 226-8501, Japan, 4IBL, Ltd., Fujioka, Gumma 375-0005, Japan, and 5Department of Physiology and 6Bioimaging Research Center, Kitasato University School of Medicine, Sagamihara, Kanagawa 252-0374, Japan In development of the peripheral nervous system, Schwann cells proliferate, migrate, and ultimately differentiate to form myelin sheath. In all of the myelination stages, Schwann cells continuously undergo morphological changes; however, little is known about their underlying molecular mechanisms. We previously cloned the dock7 gene encoding the atypical Rho family guanine-nucleotide exchange factor (GEF) and reported the positive role of Dock7, the target Rho GTPases Rac/Cdc42, and the downstream c-Jun N-terminal kinase in Schwann cell migration (Yamauchi et al., 2008). We investigated the role of Dock7 in Schwann cell differentiation and myelination. Knockdown of Dock7 by the specific small interfering (si)RNA in primary Schwann cells promotes dibutyryl cAMP-induced morpholog- ical differentiation, indicating the negative role of Dock7 in Schwann cell differentiation. It also results in a shorter duration of activation of Rac/Cdc42 and JNK, which is the negative regulator of myelination, and the earlier activation of Rho and Rho-kinase, which is the positive regulator of myelination. -

Related Malignant Phenotypes in the Nf1-Deficient MPNST
Published OnlineFirst February 19, 2013; DOI: 10.1158/1541-7786.MCR-12-0593 Molecular Cancer Genomics Research RAS/MEK–Independent Gene Expression Reveals BMP2- Related Malignant Phenotypes in the Nf1-Deficient MPNST Daochun Sun1, Ramsi Haddad2,3, Janice M. Kraniak2, Steven D. Horne1, and Michael A. Tainsky1,2 Abstract Malignant peripheral nerve sheath tumor (MPNST) is a type of soft tissue sarcoma that occurs in carriers of germline mutations in Nf1 gene as well as sporadically. Neurofibromin, encoded by the Nf1 gene, functions as a GTPase-activating protein (GAP) whose mutation leads to activation of wt-RAS and mitogen-activated protein kinase (MAPK) signaling in neurofibromatosis type I (NF1) patients' tumors. However, therapeutic targeting of RAS and MAPK have had limited success in this disease. In this study, we modulated NRAS, mitogen-activated protein/extracellular signal–regulated kinase (MEK)1/2, and neurofibromin levels in MPNST cells and determined gene expression changes to evaluate the regulation of signaling pathways in MPNST cells. Gene expression changes due to neurofibromin modulation but independent of NRAS and MEK1/2 regulation in MPNST cells indicated bone morphogenetic protein 2 (Bmp2) signaling as a key pathway. The BMP2-SMAD1/5/8 pathway was activated in NF1-associated MPNST cells and inhibition of BMP2 signaling by LDN-193189 or short hairpin RNA (shRNA) to BMP2 decreased the motility and invasion of NF1-associated MPNST cells. The pathway-specific gene changes provide a greater understanding of the complex role of neurofibromin in MPNST pathology and novel targets for drug discovery. Mol Cancer Res; 11(6); 616–27. -

Paxillin: a Focal Adhesion-Associated Adaptor Protein
Oncogene (2001) 20, 6459 ± 6472 ã 2001 Nature Publishing Group All rights reserved 0950 ± 9232/01 $15.00 www.nature.com/onc Paxillin: a focal adhesion-associated adaptor protein Michael D Schaller*,1 1Department of Cell and Developmental Biology, Lineberger Comprehensive Cancer Center and Comprehensive Center for In¯ammatory Disorders, University of North Carolina, Chapel Hill, North Carolina, NC 27599, USA Paxillin is a focal adhesion-associated, phosphotyrosine- The molecular cloning of paxillin revealed a number containing protein that may play a role in several of motifs that are now known to function in mediating signaling pathways. Paxillin contains a number of motifs protein ± protein interactions (see Figure 1) (Turner that mediate protein ± protein interactions, including LD and Miller, 1994; Salgia et al., 1995a). The N-terminal motifs, LIM domains, an SH3 domain-binding site and half of paxillin contains a proline-rich region that SH2 domain-binding sites. These motifs serve as docking could serve as an SH3 domain-binding site. Several sites for cytoskeletal proteins, tyrosine kinases, serine/ tyrosine residues conforming to SH2 domain binding threonine kinases, GTPase activating proteins and other sites were also noted. In addition, the N-terminal adaptor proteins that recruit additional enzymes into domain of paxillin contains ®ve copies of a peptide complex with paxillin. Thus paxillin itself serves as a sequence, called the LD motif, which are now known docking protein to recruit signaling molecules to a to function as binding sites for other proteins (see speci®c cellular compartment, the focal adhesions, and/ Table 1) (Brown et al., 1998a). The C-terminal half of or to recruit speci®c combinations of signaling molecules paxillin is comprised of four LIM domains, which are into a complex to coordinate downstream signaling. -

Cdc42 and Rac1 Activity Is Reduced in Human Pheochromocytoma and Correlates with FARP1 and ARHGEF1 Expression
234 P Croisé et al. Rho-GTPase activity in human 23:4 281–293 Research pheochromocytoma Cdc42 and Rac1 activity is reduced in human pheochromocytoma and correlates with FARP1 and ARHGEF1 expression Pauline Croisé1, Sébastien Houy1, Mathieu Gand1, Joël Lanoix2, Valérie Calco1, Petra Tóth1, Laurent Brunaud3, Sandra Lomazzi4, Eustache Paramithiotis2, Daniel Chelsky2, Stéphane Ory1,* and Stéphane Gasman1,* Correspondence 1Institut des Neurosciences Cellulaires et Intégratives (INCI), CNRS UPR 3212, Strasbourg, France should be addressed 2Caprion Proteome, Inc., Montréal, Québec, Canada to S Ory or S Gasman 3Service de Chirurgie Digestive, Hépato-bilaire et Endocrinienne, CHRU Nancy, Hôpitaux de Brabois, Email Vandoeuvre les Nancy, France [email protected] or 4Centre de Ressources Biologiques (CRB), CHRU Nancy, Hôpitaux de Brabois, Vandoeuvres les Nancy, France [email protected] *(S Ory and S Gasman contributed equally to this work) Abstract Among small GTPases from the Rho family, Cdc42, Rac, and Rho are well known to mediate Key Words a large variety of cellular processes linked with cancer biology through their ability to cycle f Rho-GTPases between an inactive (GDP-bound) and an active (GTP-bound) state. Guanine nucleotide f pheochromocytoma exchange factors (GEFs) stimulate the exchange of GDP for GTP to generate the activated f mass spectrometry form, whereas the GTPase-activating proteins (GAPs) catalyze GTP hydrolysis, leading to f Rho-GEF Endocrine-Related Cancer Endocrine-Related the inactivated form. Modulation of Rho GTPase activity following altered expression of Rho-GEFs and/or Rho-GAPs has already been reported in various human tumors. However, nothing is known about the Rho GTPase activity or the expression of their regulators in human pheochromocytomas, a neuroendocrine tumor (NET) arising from chromaffin cells of the adrenal medulla. -

Role of Excess Inorganic Pyrophosphate in Primer-Extension Genotyping Assays
Methods Role of Excess Inorganic Pyrophosphate in Primer-Extension Genotyping Assays Ming Xiao,1 Angie Phong,1 Kristen L. Lum,1 Richard A. Greene,2 Philip R. Buzby,2,3 and Pui-Yan Kwok1,4,5 1Cardiovascular Research Institute, University of California, San Francisco, San Francisco, California 94143-0130, USA; 2PerkinElmer Life and Analytical Sciences, Inc., Boston, Massachusetts 02118-2512, USA We have developed and genotyped >15,000 SNP assays by using a primer extension genotyping assay with fluorescence polarization (FP) detection. Although the 80% success rate of this assay is similar to those of other SNP genotyping assays, we wanted to determine the reasons for the failures and find ways to improve the assay. We observed that the failed assays fell into three general patterns: PCR failure, excess of heterozygous genotypes, and loss of FP signal for one of the dye labels. After analyzing several hundred failed assays, we concluded that 5% of the assays had PCR failure and had to be redesigned. We also discovered that the other two categories of failures were due to misincorporation of one of the dye-terminators during the primer extension reaction as a result of primer shortening with a reverse reaction involving inorganic pyrophosphate, and to the quenching of R110-terminator after its incorporation onto the SNP primer. The relatively slow incorporation of R110 acycloterminators by AcycloPol compounds the problem with the R110 label. In this report, we describe the source of the problems and simple ways to correct these problems by adding pyrophosphatase, using quenching as part of the analysis, and replacing R110 by Texas red as one of the dye labels. -

Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding Rnas
Review Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding RNAs Yu Zhang †, Jun Li †, Xing-Ning Lai, Xue-Qiao Jiao, Jun-Ping Xiong and Li-Xia Xiong * Department of Pathophysiology, Jiangxi Province Key Laboratory of Tumor Pathogenesis and Molecular Pathology, Medical College, Nanchang University, 461 Bayi Road, Nanchang 330006, China; [email protected] (Y.Z.); [email protected] (J.L.); [email protected] (X.-N.L.); [email protected] (X.-Q.J.); [email protected] (J.-P.X.) * Correspondence: [email protected]; Tel.: +86-791-8636-0556 † These authors contributed equally to this work. Received: 30 December 2018; Accepted: 8 February 2019; Published: 11 February 2019 Abstract: Breast cancer is the most common malignant tumors in females. Although the conventional treatment has demonstrated a certain effect, some limitations still exist. The Rho guanosine triphosphatase (GTPase) Cdc42 (Cell division control protein 42 homolog) is often upregulated by some cell surface receptors and oncogenes in breast cancer. Cdc42 switches from inactive guanosine diphosphate (GDP)-bound to active GTP-bound though guanine-nucleotide- exchange factors (GEFs), results in activation of signaling cascades that regulate various cellular processes such as cytoskeletal changes, proliferation and polarity establishment. Targeting Cdc42 also provides a strategy for precise breast cancer therapy. In addition, Cdc42 is a potential target for several types of non-coding RNAs including microRNAs and lncRNAs. These non-coding RNAs is extensively involved in Cdc42-induced tumor processes, while many of them are aberrantly expressed. Here, we focus on the role of Cdc42 in cell morphogenesis, proliferation, motility, angiogenesis and survival, introduce the Cdc42-targeted non-coding RNAs, as well as present current development of effective Cdc42-targeted inhibitors in breast cancer. -

Redefining the Specificity of Phosphoinositide-Binding by Human
bioRxiv preprint doi: https://doi.org/10.1101/2020.06.20.163253; this version posted June 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Redefining the specificity of phosphoinositide-binding by human PH domain-containing proteins Nilmani Singh1†, Adriana Reyes-Ordoñez1†, Michael A. Compagnone1, Jesus F. Moreno Castillo1, Benjamin J. Leslie2, Taekjip Ha2,3,4,5, Jie Chen1* 1Department of Cell & Developmental Biology, University of Illinois at Urbana-Champaign, Urbana, IL 61801; 2Department of Biophysics and Biophysical Chemistry, Johns Hopkins University School of Medicine, Baltimore, MD 21205; 3Department of Biophysics, Johns Hopkins University, Baltimore, MD 21218; 4Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD 21205; 5Howard Hughes Medical Institute, Baltimore, MD 21205, USA †These authors contributed equally to this work. *Correspondence: [email protected]. bioRxiv preprint doi: https://doi.org/10.1101/2020.06.20.163253; this version posted June 21, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. ABSTRACT Pleckstrin homology (PH) domains are presumed to bind phosphoinositides (PIPs), but specific interaction with and regulation by PIPs for most PH domain-containing proteins are unclear. Here we employed a single-molecule pulldown assay to study interactions of lipid vesicles with full-length proteins in mammalian whole cell lysates. -

A Rac/Cdc42 Exchange Factor Complex Promotes Formation of Lateral filopodia and Blood Vessel Lumen Morphogenesis
ARTICLE Received 1 Oct 2014 | Accepted 26 Apr 2015 | Published 1 Jul 2015 DOI: 10.1038/ncomms8286 OPEN A Rac/Cdc42 exchange factor complex promotes formation of lateral filopodia and blood vessel lumen morphogenesis Sabu Abraham1,w,*, Margherita Scarcia2,w,*, Richard D. Bagshaw3,w,*, Kathryn McMahon2,w, Gary Grant2, Tracey Harvey2,w, Maggie Yeo1, Filomena O.G. Esteves2, Helene H. Thygesen2,w, Pamela F. Jones4, Valerie Speirs2, Andrew M. Hanby2, Peter J. Selby2, Mihaela Lorger2, T. Neil Dear4,w, Tony Pawson3,z, Christopher J. Marshall1 & Georgia Mavria2 During angiogenesis, Rho-GTPases influence endothelial cell migration and cell–cell adhesion; however it is not known whether they control formation of vessel lumens, which are essential for blood flow. Here, using an organotypic system that recapitulates distinct stages of VEGF-dependent angiogenesis, we show that lumen formation requires early cytoskeletal remodelling and lateral cell–cell contacts, mediated through the RAC1 guanine nucleotide exchange factor (GEF) DOCK4 (dedicator of cytokinesis 4). DOCK4 signalling is necessary for lateral filopodial protrusions and tubule remodelling prior to lumen formation, whereas proximal, tip filopodia persist in the absence of DOCK4. VEGF-dependent Rac activation via DOCK4 is necessary for CDC42 activation to signal filopodia formation and depends on the activation of RHOG through the RHOG GEF, SGEF. VEGF promotes interaction of DOCK4 with the CDC42 GEF DOCK9. These studies identify a novel Rho-family GTPase activation cascade for the formation of endothelial cell filopodial protrusions necessary for tubule remodelling, thereby influencing subsequent stages of lumen morphogenesis. 1 Institute of Cancer Research, Division of Cancer Biology, 237 Fulham Road, London SW3 6JB, UK. -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of