A Phylogenomic Perspective on the Radiation of Ray-Finned Fishes Based Upon Targeted Sequencing of Ultraconserved Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters
Copeia, 2007(3), pp. 520–533 The Phylogeny of Oncorhynchus (Euteleostei: Salmonidae) Based on Behavioral and Life History Characters MANU ESTEVE AND DEBORAH A. MCLENNAN There is no consensus between morphological and molecular data concerning the relationships within the Pacific basin salmon and trout clade Oncorhynchus. In this paper we add another source of characters to the discussion. Phylogenetic analysis of 39 behavioral and life history traits produced one tree structured (O. clarki (O. mykiss (O. masou (O. kisutch (O. tshawytscha (O. nerka (O. keta, O. gorbuscha))))))). This topology is congruent with the phylogeny based upon Bayesian analysis of all available nuclear and mitochondrial gene sequences, with the exception of two nodes: behavior supports the morphological data in breaking the sister-group relationship between O. mykiss and O. clarki, and between O. kisutch and O. tshawytscha. The behavioral traits agreed with molecular rather than morphological data in placing O. keta as the sister-group of O. gorbuscha. The behavioral traits also resolve the molecular-based ambiguity concerning the placement of O. masou, placing it as sister to the rest of the Pacific basin salmon. Behavioral plus morphological data placed Salmo, not Salvelinus, as more closely related to Oncorhynchus, but that placement was only weakly supported and awaits collection of missing data from enigmatic species such as the lake trout, Salvelinus namaycush. Overall, the phenotypic characters helped resolve ambiguities that may have been created by molecular introgression, while the molecular traits helped resolve ambiguities introduced by phenotypic homoplasy. It seems reasonable therefore, that systematists can best respond to the escalating biodiversity crisis by forming research groups to gather behavioral and ecological information while specimens are being collected for morphological and molecular analysis. -

Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi
Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 (http://www.accessscience.com/) Article by: Boschung, Herbert Department of Biological Sciences, University of Alabama, Tuscaloosa, Alabama. Gardiner, Brian Linnean Society of London, Burlington House, Piccadilly, London, United Kingdom. Publication year: 2014 DOI: http://dx.doi.org/10.1036/1097-8542.680400 (http://dx.doi.org/10.1036/1097-8542.680400) Content Morphology Euteleostei Bibliography Phylogeny Classification Additional Readings Clupeomorpha and Ostariophysi The most recent group of actinopterygians (rayfin fishes), first appearing in the Upper Triassic (Fig. 1). About 26,840 species are contained within the Teleostei, accounting for more than half of all living vertebrates and over 96% of all living fishes. Teleosts comprise 517 families, of which 69 are extinct, leaving 448 extant families; of these, about 43% have no fossil record. See also: Actinopterygii (/content/actinopterygii/009100); Osteichthyes (/content/osteichthyes/478500) Fig. 1 Cladogram showing the relationships of the extant teleosts with the other extant actinopterygians. (J. S. Nelson, Fishes of the World, 4th ed., Wiley, New York, 2006) 1 of 9 10/7/2015 1:07 PM Teleostei - AccessScience from McGraw-Hill Education http://www.accessscience.com/content/teleostei/680400 Morphology Much of the evidence for teleost monophyly (evolving from a common ancestral form) and relationships comes from the caudal skeleton and concomitant acquisition of a homocercal tail (upper and lower lobes of the caudal fin are symmetrical). This type of tail primitively results from an ontogenetic fusion of centra (bodies of vertebrae) and the possession of paired bracing bones located bilaterally along the dorsal region of the caudal skeleton, derived ontogenetically from the neural arches (uroneurals) of the ural (tail) centra. -

Black Marlin (Makaira Indica) Blue Marlin (Makaira Nigricans) Blue
Black marlin (Makaira indica) Blue marlin (Makaira nigricans) Blue shark (Prionace glauca) Opah (Lampris guatus) Shorin mako shark (Isurus oxyrinchus) Striped marlin (Kaijkia audax) Western Central Pacific, North Pacific, South Pacific Pelagic longline July 12, 2016 Alexia Morgan, Consulng Researcher Disclaimer Seafood Watch® strives to have all Seafood Reports reviewed for accuracy and completeness by external sciensts with experse in ecology, fisheries science and aquaculture. Scienfic review, however, does not constute an endorsement of the Seafood Watch® program or its recommendaons on the part of the reviewing sciensts. Seafood Watch® is solely responsible for the conclusions reached in this report. Table of Contents Table of Contents 2 About Seafood Watch 3 Guiding Principles 4 Summary 5 Final Seafood Recommendations 5 Introduction 8 Assessment 10 Criterion 1: Impacts on the species under assessment 10 Criterion 2: Impacts on other species 22 Criterion 3: Management Effectiveness 45 Criterion 4: Impacts on the habitat and ecosystem 55 Acknowledgements 58 References 59 Appendix A: Extra By Catch Species 69 2 About Seafood Watch Monterey Bay Aquarium’s Seafood Watch® program evaluates the ecological sustainability of wild-caught and farmed seafood commonly found in the United States marketplace. Seafood Watch® defines sustainable seafood as originang from sources, whether wild-caught or farmed, which can maintain or increase producon in the long-term without jeopardizing the structure or funcon of affected ecosystems. Seafood Watch® makes its science-based recommendaons available to the public in the form of regional pocket guides that can be downloaded from www.seafoodwatch.org. The program’s goals are to raise awareness of important ocean conservaon issues and empower seafood consumers and businesses to make choices for healthy oceans. -

1 Exon Probe Sets and Bioinformatics Pipelines for All Levels of Fish Phylogenomics
bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Exon probe sets and bioinformatics pipelines for all levels of fish phylogenomics 2 3 Lily C. Hughes1,2,3,*, Guillermo Ortí1,3, Hadeel Saad1, Chenhong Li4, William T. White5, Carole 4 C. Baldwin3, Keith A. Crandall1,2, Dahiana Arcila3,6,7, and Ricardo Betancur-R.7 5 6 1 Department of Biological Sciences, George Washington University, Washington, D.C., U.S.A. 7 2 Computational Biology Institute, Milken Institute of Public Health, George Washington 8 University, Washington, D.C., U.S.A. 9 3 Department of Vertebrate Zoology, National Museum of Natural History, Smithsonian 10 Institution, Washington, D.C., U.S.A. 11 4 College of Fisheries and Life Sciences, Shanghai Ocean University, Shanghai, China 12 5 CSIRO Australian National Fish Collection, National Research Collections of Australia, 13 Hobart, TAS, Australia 14 6 Sam Noble Oklahoma Museum of Natural History, Norman, O.K., U.S.A. 15 7 Department of Biology, University of Oklahoma, Norman, O.K., U.S.A. 16 17 *Corresponding author: Lily C. Hughes, [email protected]. 18 Current address: Department of Organismal Biology and Anatomy, University of Chicago, 19 Chicago, IL. 20 21 Keywords: Actinopterygii, Protein coding, Systematics, Phylogenetics, Evolution, Target 22 capture 23 1 bioRxiv preprint doi: https://doi.org/10.1101/2020.02.18.949735; this version posted February 19, 2020. -
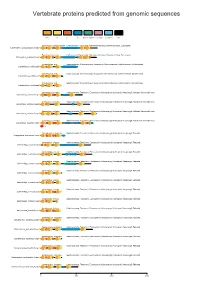
Vertebrate Proteins Predicted from Genomic Sequences
Vertebrate proteins predicted from genomic sequences VWD C8 TIL PTS Mucin2_WxxW F5_F8_type_C FCGBP_N VWC Lethenteron_camtschaticum Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Lethenteron Lethenteron_camtschaticum.0.pep1 Petromyzon_marinus Cyclostomata; Hyperoartia; Petromyzontiformes; Petromyzontidae; Petromyzon Petromyzon_marinus.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep1 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep2 Callorhinchus_milii Gnathostomata; Chondrichthyes; Holocephali; Chimaeriformes; Callorhinchidae; Callorhinchus Callorhinchus_milii.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep1 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep2 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.0.pep3 Lepisosteus_oculatus Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Holostei; Semionotiformes; Lepisosteus_oculatus.1.pep1 TILa Cynoglossus_semilaevis Gnathostomata; Teleostomi; Euteleostomi; Actinopterygii; Actinopteri; Neopterygii; Teleostei; Cynoglossus_semilaevis.1.pep1 -
Transcriptome Ortholog Alignment Sequence Tools (TOAST) for Phylogenomic Dataset Assembly
Transcriptome Ortholog Alignment Sequence Tools (TOAST) for Phylogenomic Dataset Assembly Dustin J. Wcisel North Carolina State University J. Thomas Howard North Carolina State University Jeffrey A. Yoder North Carolina State University Alex Dornburg ( [email protected] ) NC Museum of Natural Sciences https://orcid.org/0000-0003-0863-2283 Software Keywords: BUSCO ortholog assembly, Cetacean and teleost sh phylogeny, Missing Data Visualization, Transcriptome, Concatenated Alignment Posted Date: March 12th, 2020 DOI: https://doi.org/10.21203/rs.2.16269/v4 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at BMC Evolutionary Biology on March 30th, 2020. See the published version at https://doi.org/10.1186/s12862-020-01603-w. Page 1/18 Abstract Background Advances in next-generation sequencing technologies have reduced the cost of whole transcriptome analyses, allowing characterization of non-model species at unprecedented levels. The rapid pace of transcriptomic sequencing has driven the public accumulation of a wealth of data for phylogenomic analyses, however lack of tools aimed towards phylogeneticists to eciently identify orthologous sequences currently hinders effective harnessing of this resource. Results We introduce TOAST, an open source R software package that can utilize the ortholog searches based on the software Benchmarking Universal Single-Copy Orthologs (BUSCO) to assemble multiple sequence alignments of orthologous loci from transcriptomes for any group of organisms. By streamlining search, query, and alignment, TOAST automates the generation of locus and concatenated alignments, and also presents a series of outputs from which users can not only explore missing data patterns across their alignments, but also reassemble alignments based on user-dened acceptable missing data levels for a given research question. -

Diaphus Taaningi Norman, the Principal Component If a Shallow Sound-Scattering Layer in the Cariaco Trench, Venezuela'
The Journal of Marine Research is an online peer-reviewed journal that publishes original research on a broad array of topics in physical, biological, and chemical oceanography. In publication since 1937, it is one of the oldest journals in American marine science and occupies a unique niche within the ocean sciences, with a rich tradition and distinguished history as part of the Sears Foundation for Marine Research at Yale University. Past and current issues are available at journalofmarineresearch.org. Yale University provides access to these materials for educational and research purposes only. Copyright or other proprietary rights to content contained in this document may be held by individuals or entities other than, or in addition to, Yale University. You are solely responsible for determining the ownership of the copyright, and for obtaining permission for your intended use. Yale University makes no warranty that your distribution, reproduction, or other use of these materials will not infringe the rights of third parties. This work is licensed under the Creative Commons Attribution- NonCommercial-ShareAlike 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. Journal of Marine Research, Sears Foundation for Marine Research, Yale University PO Box 208118, New Haven, CT 06520-8118 USA (203) 432-3154 fax (203) 432-5872 [email protected] www.journalofmarineresearch.org Diaphus taaningi Norman, the Principal Component if a Shallow Sound-Scattering Layer in the Cariaco Trench, Venezuela' Ronald C. Baird University of South Florida Department of Marine Science St. -

Table S1.Xlsx
Bone type Bone type Taxonomy Order/series Family Valid binomial Outdated binomial Notes Reference(s) (skeletal bone) (scales) Actinopterygii Incertae sedis Incertae sedis Incertae sedis †Birgeria stensioei cellular this study †Birgeria groenlandica cellular Ørvig, 1978 †Eurynotus crenatus cellular Goodrich, 1907; Schultze, 2016 †Mimipiscis toombsi †Mimia toombsi cellular Richter & Smith, 1995 †Moythomasia sp. cellular cellular Sire et al., 2009; Schultze, 2016 †Cheirolepidiformes †Cheirolepididae †Cheirolepis canadensis cellular cellular Goodrich, 1907; Sire et al., 2009; Zylberberg et al., 2016; Meunier et al. 2018a; this study Cladistia Polypteriformes Polypteridae †Bawitius sp. cellular Meunier et al., 2016 †Dajetella sudamericana cellular cellular Gayet & Meunier, 1992 Erpetoichthys calabaricus Calamoichthys sp. cellular Moss, 1961a; this study †Pollia suarezi cellular cellular Meunier & Gayet, 1996 Polypterus bichir cellular cellular Kölliker, 1859; Stéphan, 1900; Goodrich, 1907; Ørvig, 1978 Polypterus delhezi cellular this study Polypterus ornatipinnis cellular Totland et al., 2011 Polypterus senegalus cellular Sire et al., 2009 Polypterus sp. cellular Moss, 1961a †Scanilepis sp. cellular Sire et al., 2009 †Scanilepis dubia cellular cellular Ørvig, 1978 †Saurichthyiformes †Saurichthyidae †Saurichthys sp. cellular Scheyer et al., 2014 Chondrostei †Chondrosteiformes †Chondrosteidae †Chondrosteus acipenseroides cellular this study Acipenseriformes Acipenseridae Acipenser baerii cellular Leprévost et al., 2017 Acipenser gueldenstaedtii -

© Iccat, 2007
A5 By-catch Species APPENDIX 5: BY-CATCH SPECIES A.5 By-catch species By-catch is the unintentional/incidental capture of non-target species during fishing operations. Different types of fisheries have different types and levels of by-catch, depending on the gear used, the time, area and depth fished, etc. Article IV of the Convention states: "the Commission shall be responsible for the study of the population of tuna and tuna-like fishes (the Scombriformes with the exception of Trichiuridae and Gempylidae and the genus Scomber) and such other species of fishes exploited in tuna fishing in the Convention area as are not under investigation by another international fishery organization". The following is a list of by-catch species recorded as being ever caught by any major tuna fishery in the Atlantic/Mediterranean. Note that the lists are qualitative and are not indicative of quantity or mortality. Thus, the presence of a species in the lists does not imply that it is caught in significant quantities, or that individuals that are caught necessarily die. Skates and rays Scientific names Common name Code LL GILL PS BB HARP TRAP OTHER Dasyatis centroura Roughtail stingray RDC X Dasyatis violacea Pelagic stingray PLS X X X X Manta birostris Manta ray RMB X X X Mobula hypostoma RMH X Mobula lucasana X Mobula mobular Devil ray RMM X X X X X Myliobatis aquila Common eagle ray MYL X X Pteuromylaeus bovinus Bull ray MPO X X Raja fullonica Shagreen ray RJF X Raja straeleni Spotted skate RFL X Rhinoptera spp Cownose ray X Torpedo nobiliana Torpedo -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Gar (Lepisosteidae)
Indiana Division of Fish and Wildlife’s Animal Information Series Gar (Lepisosteidae) Gar species found in Indiana waters: -Longnose Gar (Lepisosteus osseus) -Shortnose Gar (Lepisosteus platostomus) -Spotted Gar (Lepisosteus oculatus) -Alligator Gar* (Atractosteus spatula) *Alligator Gar (Atractosteus spatula) Alligator gar were extirpated in many states due to habitat destruction, but now they have been reintroduced to their old native habitat in the states of Illinois, Missouri, Arkansas, and Kentucky. Because they have been stocked into the Ohio River, there is a possibility that alligator gar are either already in Indiana or will be found here in the future. Alligator gar are one of the largest freshwater fishes of North America and can reach up to 10 feet long and weigh 300 pounds. Alligator gar are passive, solitary fishes that live in large rivers, swamps, bayous, and lakes. They have a short, wide snout and a double row of teeth on the upper jaw. They are ambush predators that eat mainly fish but have also been seen to eat waterfowl. They are not, however, harmful to humans, as they will only attack an animal that they can swallow whole. Photo Credit: Duane Raver, USFWS Other Names -garpike, billy gar -Shortnose gar: shortbill gar, stubnose gar -Longnose gar: needlenose gar, billfish Why are they called gar? The Anglo-Saxon word gar means spear, which describes the fishes’ long spear-like appearance. The genus name Lepisosteus contains the Greek words lepis which means “scale” and osteon which means “bone.” What do they look like? Gar are slender, cylindrical fishes with hard, diamond-shaped and non-overlapping scales. -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................