Hemolytic Disease of the Newborn
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Fetal Center SPRING 2019 JOURNAL in This Issue
The Fetal Center SPRING 2019 JOURNAL In This Issue FEATURES: TAKING THE RESEARCH LONG VIEW TOWARD PREVENTION OF RHESUS DISEASE 06 A New Clinical Trial: Umbilical Cord Blood Mononuclear Cells for Hypoxic Neurologic Injury 01 Leaders in Innovation: Rhesus Disease in Infants with Congenital Diaphragmatic Hernia Diagnosis and Treatment NEWS OF NOTE 04 A Miracle Baby for the Pinedas 08 The Fetal Center Welcomes New Recruits 09 NAFTNet Leadership Contact Us THE FETAL CENTER AT CHILDREN’S MEMORIAL HERMANN HOSPITAL UT Physicians Professional Building 6410 Fannin, Suite 210 Houston, TX 77030 Phone: 832.325.7288 Fax: 713.383.1464 Email: [email protected] Located within the Texas Medical Center, The Fetal Center is affiliated with Children’s ★FETAL Memorial Hermann Hospital, McGovern Medical NR School at UTHealth, and UT Physicians. To view The Fetal Center’s online resources, visit childrens.memorialhermann.org/thefetalcenter. FEATURE Leaders in Innovation: Rhesus Disease Diagnosis and Treatment Rhesus (Rh) disease, also known as Reproductive Sciences and the department of Rh-induced hemolytic disease of the Pediatric Surgery at McGovern Medical School at UTHealth. “The antibodies don’t usually cause fetus and newborn (HDFN), rhesus problems during a first pregnancy, because the baby alloimmunization or erythroblastosis may be born before the level of antibodies is high enough to have an effect. Rh antibodies are more fetalis, is relatively rare, occurring in likely to cause problems in second or later pregnan- about 2.5 out of every 100,000 live cies if the baby is Rh positive. Rh antibodies cross the placenta and attack the baby’s red blood cells, births in countries with well-established causing hemolytic anemia in the baby and leading to healthcare infrastructures. -

Transfusion Problems in Hemolytic Anemias*
Transfusion Problems in Hemolytic Anemias* ALI A. HOSSAIN! Department of Pathdlogy, Medical College of Virginia, Richmond 23219 All hemolytic anemias feature shortened red cell Extrinsic Mechanisms survival due to premature hemolysis of the cell. For the Those hemolytic anemias which are due to extrinsic purposes of this presentation, we may classify the factors may be classified, further, as non-immune or hemolytic anemias, most broadly, according to the immune. Non-immune mechanisms include a) drugs mechanisms leading to hemolysis. and chemicals (phenylhydrazine, naphthalene, lead, snake venoms); b) physical agents (heat); c) bacteria Intrinsic Mechanisms and parasites (hemolytic streptococci, Clostridium Hemolytic anemias due to intrinsically defective welchii, Bartonella, plasmodia); and d) acquired sen erythrocytes are essentially of three types. First are sitivity to penicillin, methylodopa, Keftin®, or fava those anemias in which the red cells are defective due plant as examples. Some of the agents in this. last to lack of an essential factor, eg, pernicious anemia group serve to lyse the cells, either through duect in relapse. The second type includes those in which action or by formation of antibodies. the red cells have an abnormal shape because of an These hemolytic anemias due to extrinsic factors inherited error in the chemical makeup of the hemo of the non-immune variety present no transfusion globin molecules; eg, sickle cells, elliptocytes, sphero problem for the Blood Bank. However, it ~~st b.e cytes, and the target -

Association Between ABO and Duffy Blood Types and Circulating Chemokines and Cytokines
Genes & Immunity (2021) 22:161–171 https://doi.org/10.1038/s41435-021-00137-5 ARTICLE Association between ABO and Duffy blood types and circulating chemokines and cytokines 1 2 3 4 5 6 Sarah C. Van Alsten ● John G. Aversa ● Loredana Santo ● M. Constanza Camargo ● Troy Kemp ● Jia Liu ● 4 7 8 Wen-Yi Huang ● Joshua Sampson ● Charles S. Rabkin Received: 11 February 2021 / Revised: 30 April 2021 / Accepted: 17 May 2021 / Published online: 8 June 2021 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply 2021, corrected publication 2021 Abstract Blood group antigens are inherited traits that may play a role in immune and inflammatory processes. We investigated associations between blood groups and circulating inflammation-related molecules in 3537 non-Hispanic white participants selected from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Whole-genome scans were used to infer blood types for 12 common antigen systems based on well-characterized single-nucleotide polymorphisms. Serum levels of 96 biomarkers were measured on multiplex fluorescent bead-based panels. We estimated marker associations with blood type using weighted linear or logistic regression models adjusted for age, sex, smoking status, and principal components of p 1234567890();,: 1234567890();,: population substructure. Bonferroni correction was used to control for multiple comparisons, with two-sided values < 0.05 considered statistically significant. Among the 1152 associations tested, 10 were statistically significant. Duffy blood type was associated with levels of CXCL6/GCP2, CXCL5/ENA78, CCL11/EOTAXIN, CXCL1/GRO, CCL2/MCP1, CCL13/ MCP4, and CCL17/TARC, whereas ABO blood type was associated with levels of sVEGFR2, sVEGFR3, and sGP130. -

Section 8: Hematology CHAPTER 47: ANEMIA
Section 8: Hematology CHAPTER 47: ANEMIA Q.1. A 56-year-old man presents with symptoms of severe dyspnea on exertion and fatigue. His laboratory values are as follows: Hemoglobin 6.0 g/dL (normal: 12–15 g/dL) Hematocrit 18% (normal: 36%–46%) RBC count 2 million/L (normal: 4–5.2 million/L) Reticulocyte count 3% (normal: 0.5%–1.5%) Which of the following caused this man’s anemia? A. Decreased red cell production B. Increased red cell destruction C. Acute blood loss (hemorrhage) D. There is insufficient information to make a determination Answer: A. This man presents with anemia and an elevated reticulocyte count which seems to suggest a hemolytic process. His reticulocyte count, however, has not been corrected for the degree of anemia he displays. This can be done by calculating his corrected reticulocyte count ([3% × (18%/45%)] = 1.2%), which is less than 2 and thus suggestive of a hypoproliferative process (decreased red cell production). Q.2. A 25-year-old man with pancytopenia undergoes bone marrow aspiration and biopsy, which reveals profound hypocellularity and virtual absence of hematopoietic cells. Cytogenetic analysis of the bone marrow does not reveal any abnormalities. Despite red blood cell and platelet transfusions, his pancytopenia worsens. Histocompatibility testing of his only sister fails to reveal a match. What would be the most appropriate course of therapy? A. Antithymocyte globulin, cyclosporine, and prednisone B. Prednisone alone C. Supportive therapy with chronic blood and platelet transfusions only D. Methotrexate and prednisone E. Bone marrow transplant Answer: A. Although supportive care with transfusions is necessary for treating this patient with aplastic anemia, most cases are not self-limited. -

Blood Product Replacement: Obstetric Hemorrhage
CMQCC OBSTETRIC HEMORRHAGE TOOLKIT Version 2.0 3/24/15 BLOOD PRODUCT REPLACEMENT: OBSTETRIC HEMORRHAGE Richard Lee, MD, Los Angeles County and University of Southern California Medical Center Laurence Shields, MD, Marian Regional Medical Center/Dignity Health Holli Mason, MD, Cedars-Sinai Medical Center Mark Rollins, MD, PhD, University of California, San Francisco Jed Gorlin, MD, Innovative Blood Resources/Memorial Blood Center, St. Paul, Minnesota Maurice Druzin, MD, Lucile Packard Children’s Hospital Stanford University Jennifer McNulty, MD, Long Beach Memorial Medical Center EXECUTIVE SUMMARY • Outcomes are improved with early and aggressive intervention. • Both emergency blood release and massive transfusion protocols should be in place. • In the setting of significant obstetric hemorrhage, resuscitation transfusion should be based on vital signs and blood loss and should not be delayed by waiting for laboratory results. • Calcium replacement will often be necessary with massive transfusion due to the citrate used for anticoagulation in blood products. • During massive transfusion resuscitation, the patient’s arterial blood gas, electrolytes, and core temperature should be monitored to guide clinical management and all transfused fluids should be warmed; direct warming of the patient should be initiated as needed to maintain euthermia and to avoid added coagulopathy. BACKGROUND AND LITERATURE REVIEW After the first several units of packed red blood cells (PRBCs) and in the face of continuing or worsening hemorrhage, aggressive transfusion therapy becomes critical. This report covers the experience with massive transfusion protocols. Lessons from military trauma units as well as civilian experience with motor vehicle accidents and massive obstetric hemorrhage have identified new principles such as earlier use of plasma (FFP/thawed plasma/plasma frozen within 24 hours/liquid plasma) and resuscitation transfusion while laboratory results are pending. -

Evaluation of Anemia Survey (NHANES III) Data- 9 10-28% of Patients Over 65 Years Are Anemic Mark Wurster, M.D., F.A.C.P
Anemia - Definition • National Health and Nutrition Examination Evaluation of Anemia Survey (NHANES III) data- 9 10-28% of patients over 65 years are anemic Mark Wurster, M.D., F.A.C.P. 9 One third of these are due to iron, folate, B12 The Ohio State University deficiency alone or in combination 9 One third are due to renal disease, or other chronic inflammatory response 9 One third are due to various primary marrow disorders, malignancies or other disorders Anemia Anemia - Definition Classification Schemes • A simplified approach to anemia, • Most common hematologic disorder emphasizing information already included • Decrease from normal levels of Hgb, Hct, RBC: in the CBC: 9 FlFemales – MHb14/dlMean Hgb = 14 g/dl; -2SD = 12 g /dl • Mean Cellular Volume (MCV) 9 Males – Mean Hgb = 15.5 g/dl; -2SD = 13.5 g/dl • Red Cell Distribution Width (RDW) • Caveat – Anemia is a syndrome, not a disease. • Retic count An abnormal Hgb or Hct should ALWAYS be investigated if confirmed on repeat testing. 1 Anemia Anemia Classification Schemes Classification Schemes • Mean Cellular Volume (MCV) • Red blood cell Distribution Width (RDW) • Decreased MCV (microcytic); < 80 fL 9 A numerical expression of • Normal MCV (normocytic); 80 – 99 fL anisocytosis, or variation in RBC size • Increased MCV (macrocytic); > 100 fL Anemia Anemia Classification Schemes Classification Schemes • Red blood cell Distribution Width (RDW) 9 Normal RDW - representing a uniform population • Red blood cell Distribution Width (RDW) of RBCs with respect to size (actually the standard deviation of red blood cell volume divided by the mean volume) 9 Normal; < or = to app. -

A Newly Recognized Blood Group in Domestic Shorthair Cats: the Mik Red Cell Antigen
J Vet Intern Med 2007;21:287–292 A Newly Recognized Blood Group in Domestic Shorthair Cats: The Mik Red Cell Antigen Nicole M. Weinstein, Marie-Claude Blais, Kimberly Harris, Donna A. Oakley, Lillian R. Aronson, and Urs Giger Background: Naturally occurring alloantibodies produced against A and B red cell antigens in cats can cause acute hemolytic transfusion reactions. Blood incompatibilities, unrelated to the AB blood group system, have also been suspected after blood transfusions through routine crossmatch testing or as a result of hemolytic transfusion reactions. Hypothesis: Incompatible crossmatch results among AB compatible cats signify the presence of a naturally occurring alloantibody against a newly identified blood antigen in a group of previously never transfused blood donor cats. The associated alloantibody is clinically important based upon a hemolytic transfusion reaction after inadvertent transfusion of red cells expressing this red cell antigen in a feline renal transplant recipient that lacks this red cell antigen. Methods: Blood donor and nonblood donor cats were evaluated for the presence of auto- and alloantibodies using direct antiglobulin and crossmatch tests, respectively, and were blood typed for AB blood group status. Both standard tube and novel gel column techniques were used. Results: Plasma from 3 of 65 cats and 1 feline renal transplant recipient caused incompatible crossmatch test results with AB compatible erythrocytes indicating these cats formed an alloantibody against a red cell antigen they lack, termed Mik. The 3 donors and the renal transplant recipient were crossmatch-compatible with one another. Tube and gel column crossmatch test results were similar. Conclusions and Clinical Importance: The absence of this novel Mik red cell antigen can be associated with naturally occurring anti-Mik alloantibodies and can elicit an acute hemolytic transfusion reaction after an AB-matched blood transfusion. -

Clinical Pathology Interpretation Barbara Horney
CLINICAL PATHOLOGY PATHOLOGIE CLINIQUE Clinical pathology interpretation Barbara Horney History, physical examination, and Table 1. Hematologic findings from a lethargic, laboratory findings 4-year-old schipperke 4-year-old, spayed female, schipperke was pre- Blood cell count Reference range A sented because of mild lethargy. Pale mucous mem- White blood cells branes were observed on physical examination. Table 1 (WBC) gives the results of the hematological examination of Total 6.0 X 109/L 6.0-17.1 X 109/L blood at Differential samples taken this time. No significant abnor- segmented 65% 3.85 X 109/L 3.6-11.5 X 109/L malities were identified on the serum biochemical neutrophils profile. eosinophils 2% 0.12 X 109/L 0.01-1.25 X 109/L lymphocytes 27% 1.59 X 109/L 1.0-4.8 X 109/L Interpretation and discussion monocytes 6% 0.35 X 109/L 0.15-1.35 X 109/L Red blood cells The hematology results can be summarized as severe, Total 1.2 X 1012/L 5.5-8.5 X 109/L microcytic, normochromic, nonregenerative anemia nucleated 1/100 WBC <1-2 per 100 WBC associated with marked spherocytosis. spherocytes 4+ microcytosis 2+ The presence of spherocytes is often associated with immune-mediated hemolytic disease [1,2], although Platelets estimated normal hereditary membrane defects [3] and zinc toxicosis [4] in number can also result in spherocyte formation. A direct antibody Reticulocytes 0 X 109/L up to 120 X 109/L test (Coomb's test) was weakly positive. This finding can Hemoglobin 22 g/L 120-180 g/L support the tentative diagnosis of anemia of immune- Hematocrit 0.068 L/L 0.37-0.55 L/L mediated etiology, although this test is subject to both Mean corpuscular false positive and false negative results [2,5]. -

Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2
animals Review Role of Infection and Immunity in Bovine Perinatal Mortality: Part 2. Fetomaternal Response to Infection and Novel Diagnostic Perspectives Paulina Jawor 1,* , John F. Mee 2 and Tadeusz Stefaniak 1 1 Department of Immunology, Pathophysiology and Veterinary Preventive Medicine, Wrocław University of Environmental and Life Sciences, 50-375 Wrocław, Poland; [email protected] 2 Animal and Bioscience Research Department, Teagasc, Moorepark Research Centre, P61 P302 Fermoy, County Cork, Ireland; [email protected] * Correspondence: [email protected] Simple Summary: Bovine perinatal mortality (death of the fetus or calf before, during, or within 48 h of calving at full term (≥260 days) may be caused by noninfectious and infectious causes. Although infectious causes of fetal mortality are diagnosed less frequently, infection in utero may also compromise the development of the fetus without causing death. This review presents fetomaternal responses to infection and the changes which can be observed in such cases. Response to infection, especially the concentration of immunoglobulins and some acute-phase proteins, may be used for diagnostic purposes. Some changes in internal organs may also be used as an indicator of infection in utero. However, in all cases (except pathogen-specific antibody response) non-pathogen-specific responses do not aid in pathogen-specific diagnosis of the cause of calf death. But, nonspecific markers of in utero infection may allow us to assign the cause of fetal mortality to infection and thus Citation: Jawor, P.; Mee, J.F.; increase our overall diagnosis rate, particularly in cases of the “unexplained stillbirth”. Stefaniak, T. Role of Infection and Immunity in Bovine Perinatal Abstract: Bovine perinatal mortality due to infection may result either from the direct effects of Mortality: Part 2. -

Anemia and the QT Interval in Hypertensive Patients
2084 International Journal of Collaborative Research on Internal Medicine & Public Health Anemia and the QT interval in hypertensive patients Ioana Mozos 1*, Corina Serban 2, Rodica Mihaescu 3 1 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 2 Department of Functional Sciences, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania 3 1st Department of Internal Medicine, “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania * Corresponding Author ; Email: [email protected] Abstract Introduction: A prolonged ECG QT interval duration and an increased QT dispersion (QTd) are predictors of sudden cardiac death. Anemia is known as a marker of adverse outcome in cardiovascular disease. Objective: The aim was to assess the relationship between anemia and QT intervals in hypertensive patients. Method: A total of 72 hypertensive patients underwent standard 12-lead ECG. QT intervals and QT dispersions were manually measured. Complete blood count was also assessed. Result: Linear regression analysis revealed significant associations between prolonged QTc and increased QTd and anemia and macrocytosis, respectively. Multiple regression analysis revealed a significant association between red cell distribution width (RDW) >15% and prolonged heart rate corrected maximal QT interval duration (QTc) and QT interval in lead DII (QTIIc). The most sensitive and specific predictor of prolonged QTc and QTIIc was anisocytosis. Anemia was the most sensitive predictor of -
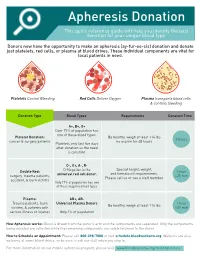
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Blood Type and Transplantation & A2 Donor to B Recipient
Page 1 of 2 Blood Type and Transplantation Information for Kidney Transplant Patients Does blood type matter in transplantation? Everyone waiting for a transplant has their blood typed. You will have one of four blood types: O, A, B or AB. Your blood type is determined by the antigens that are present on your blood cells. These antigens are A or B. These antigens will be found both in your blood and on your organs. What antigen does each blood type have? Blood type O Blood type A Blood type B Blood type AB have no have A antigens. have B antigens. have both A O antigens. A B AB and B antigens. How does my body react to antigens? Your body will react to antigens that are different than your own by attacking with antibodies. Antibodies are proteins created by your immune system to attack anything that does not belong. Antibodies are the soldiers in your body’s army protecting you from foreign invasions such as viruses. Unfortunately, the antibodies cannot tell the difference between harmful viruses and beneficial transplanted organs. What blood type will my donor be? Transplants can occur between all blood types. However, when the donor’s blood type is different than yours and there are different antigens being transplanted on your new organ, your antibodies will be triggered and attack the transplanted organ. This is called rejection. Because of this, transplants usually happen between a donor and a recipient of the same blood type. This is called an identical transplant. Can I get an organ from a donor that has a different blood type than mine? Yes! If you do not have antibodies in your body against the antigens that come from the donor, your immune system should not attack the transplanted organ.