Blood Product Replacement: Obstetric Hemorrhage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stem Cells and Artificial Substitutes Could Ease the Dependence on Blood Donations
OUTLOOK BLOOD DOING WITHOUT DONORS Stem cells and artificial substitutes could ease the dependence on blood donations. ANDREW BAKER BY ELIE DOLGIN S12 | NATURE | VOL 549 | 28 SEPTEMBER©2017 Ma c2017millan Publishers Li mited, part of Spri nger Nature. All ri ghts reserved. ©2017 Mac millan Publishers Li mited, part of Spri nger Nature. All ri ghts reserved. BLOOD OUTLOOK ach year, at about 13,000 collection centres worldwide, phlebotomists stick needles in the veins of healthy vol- unteers and amass in excess of 110 million donations of blood. The volume collected is enough to fill 20 Olympic- sized swimming pools — but it’s nowhere near to meeting the medical demand for whole blood or its components. To fill the gap, an enterprising group of stem-cell biologists and bio- Eengineers hopes to produce a safe, reliable and bottomless supply of on-demand blood substitutes in the laboratory. According to Robert Lanza, a pioneer of stem cell therapies and head IMAGES IWM VIA GETTY CHETWYN/ SGT. of global regenerative medicine at Astellas Pharma in Marlborough, Massachusetts, current technologies are not yet ready to compete with the real stuff. “We’re not going to put blood banks out of business any time soon,” he says. But in the near future, artificial blood products could be approved for use when transfusions are not otherwise an option, such as during combat or in people with a religious objection to receiving blood transfusions. And therapies that rely on reprogrammed stem cells to produce components of blood might also help transfusion centres to relieve shortages or to avoid donor-derived contamination. -

Hemolytic Disease of the Newborn
Intensive Care Nursery House Staff Manual Hemolytic Disease of the Newborn INTRODUCTION and DEFINITION: Hemolytic Disease of the Newborn (HDN), also known as erythroblastosis fetalis, isoimmunization, or blood group incompatibility, occurs when fetal red blood cells (RBCs), which possess an antigen that the mother lacks, cross the placenta into the maternal circulation, where they stimulate antibody production. The antibodies return to the fetal circulation and result in RBC destruction. DIFFERENTIAL DIAGNOSIS of hemolytic anemia in a newborn infant: -Isoimmunization -RBC enzyme disorders (e.g., G6PD, pyruvate kinase deficiency) -Hemoglobin synthesis disorders (e.g., alpha-thalassemias) -RBC membrane abnormalities (e.g., hereditary spherocytosis, elliptocytosis) -Hemangiomas (Kasabach Merritt syndrome) -Acquired conditions, such as sepsis, infections with TORCH or Parvovirus B19 (anemia due to RBC aplasia) and hemolysis secondary to drugs. ISOIMMUNIZATION A. Rh disease (Rh = Rhesus factor) (1) Genetics: Rh positive (+) denotes presence of D antigen. The number of antigenic sites on RBCs varies with genotype. Prevalence of genotype varies with the population. Rh negative (d/d) individuals comprise 15% of Caucasians, 5.5% of African Americans, and <1% of Asians. A sensitized Rh negative mother produces anti-Rh IgG antibodies that cross the placenta. Risk factors for antibody production include 2nd (or later) pregnancies*, maternal toxemia, paternal zygosity (D/D rather than D/d), feto-maternal compatibility in ABO system and antigen load. (2) Clinical presentation of HDN varies from mild jaundice and anemia to hydrops fetalis (with ascites, pleural and pericardial effusions). Because the placenta clears bilirubin, the chief risk to the fetus is anemia. Extramedullary hematopoiesis (due to anemia) results in hepatosplenomegaly. -

Association Between ABO and Duffy Blood Types and Circulating Chemokines and Cytokines
Genes & Immunity (2021) 22:161–171 https://doi.org/10.1038/s41435-021-00137-5 ARTICLE Association between ABO and Duffy blood types and circulating chemokines and cytokines 1 2 3 4 5 6 Sarah C. Van Alsten ● John G. Aversa ● Loredana Santo ● M. Constanza Camargo ● Troy Kemp ● Jia Liu ● 4 7 8 Wen-Yi Huang ● Joshua Sampson ● Charles S. Rabkin Received: 11 February 2021 / Revised: 30 April 2021 / Accepted: 17 May 2021 / Published online: 8 June 2021 This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply 2021, corrected publication 2021 Abstract Blood group antigens are inherited traits that may play a role in immune and inflammatory processes. We investigated associations between blood groups and circulating inflammation-related molecules in 3537 non-Hispanic white participants selected from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Whole-genome scans were used to infer blood types for 12 common antigen systems based on well-characterized single-nucleotide polymorphisms. Serum levels of 96 biomarkers were measured on multiplex fluorescent bead-based panels. We estimated marker associations with blood type using weighted linear or logistic regression models adjusted for age, sex, smoking status, and principal components of p 1234567890();,: 1234567890();,: population substructure. Bonferroni correction was used to control for multiple comparisons, with two-sided values < 0.05 considered statistically significant. Among the 1152 associations tested, 10 were statistically significant. Duffy blood type was associated with levels of CXCL6/GCP2, CXCL5/ENA78, CCL11/EOTAXIN, CXCL1/GRO, CCL2/MCP1, CCL13/ MCP4, and CCL17/TARC, whereas ABO blood type was associated with levels of sVEGFR2, sVEGFR3, and sGP130. -

Platelet-Rich Plasmapheresis: a Meta-Analysis of Clinical Outcomes and Costs
THE jOURNAL OF EXTRA-CORPOREAL TECHNOLOGY Original Article Platelet-Rich Plasmapheresis: A Meta-Analysis of Clinical Outcomes and Costs Chris Brown Mahoney , PhD Industrial Relations Center, Carlson School of Management, University of Minnesota, Minneapolis, MN Keywords: platelet-rich plasmapheresis, sequestration, cardiopulmonary bypass, outcomes, economics, meta-analysis Presented at the American Society of Extra-Corporeal Technology 35th International Conference, April 3-6, 1997, Phoenix, Arizona ABSTRACT Platelet-rich plasmapheresis (PRP) just prior to cardiopulmonary bypass (CPB) surgery is used to improve post CPB hemostasis and to minimize the risks associated with exposure to allogeneic blood and its components. Meta-analysis examines evidence ofPRP's impact on clinical outcomes by integrating the results across published research studies. Data on clinical outcomes was collected from 20 pub lished studies. These outcomes, DRG payment rates, and current national average costs were used to examine the impact of PRP on costs. This study provides evidence that the use of PRP results in improved clinical outcomes when compared to the identical control groups not receiving PRP. These improved clinical out comes result in subsequent lower costs per patient in the PRP groups. All clinical outcomes analyzed were improved: blood product usage, length of stay, intensive care stay, time to extu bation, incidence of cardiovascular accident, and incidence of reoperation. The most striking differences occur in use of all blood products, particularly packed red blood cells. This study provides an example of how initial expenditure on technology used during CPB results in overall cost savings. Estimated cost savings range from $2,505.00 to $4,209.00. -
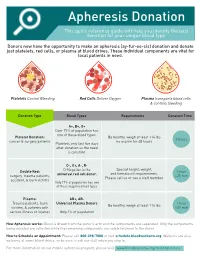
Apheresis Donation This Quick Reference Guide Will Help You Identify the Best Donation for Your Unique Blood Type
Apheresis Donation This quick reference guide will help you identify the best donation for your unique blood type. Donors now have the opportunity to make an apheresis (ay-fur-ee-sis) donation and donate just platelets, red cells, or plasma at blood drives. These individual components are vital for local patients in need. Platelets Control Bleeding Red Cells Deliver Oxygen Plasma transports blood cells & controls bleeding Donation Type Blood Types Requirements Donation Time A+, B+, O+ Over 75% of population has one of these blood types. Platelet Donation: Be healthy, weigh at least 114 lbs 2 hours cancer & surgery patients no aspirin for 48 hours Platelets only last five days after donation so the need is constant. O-, O+, A-, B- Special height, weight, Double Red: O-Negative is the 1 hour and hematocrit requirements. surgery, trauma patients, universal red cell donor. +25 min Please call us or see a staff member accident, & burn victims Only 17% of population has one of these negative blood types Plasma: AB+, AB- Trauma patients, burn Universal Plasma Donors 1 hour Be healthy, weigh at least 114 lbs victims, & patients with +30 min serious illness or injuries Only 4% of population How Apheresis works: Blood is drawn from the donor’s arm and the components are separated. Only the components being donated are collected while the remaining components are safely returned to the donor How to Schedule an Appointment: Please call 800-398-7888 or visit schedule.bloodworksnw.org. Walk-ins are also welcome at some blood drives, so be sure to ask our staff when you stop in. -

Blood Product Modifications: Leukofiltration, Irradiation and Washing
Blood Product Modifications: Leukofiltration, Irradiation and Washing 1. Leukocyte Reduction Definitions and Standards: o Process also known as leukoreduction, or leukofiltration o Applicable AABB Standards, 25th ed. Leukocyte-reduced RBCs At least 85% of original RBCs < 5 x 106 WBCs in 95% of units tested . Leukocyte-reduced Platelet Concentrates: At least 5.5 x 1010 platelets in 75% of units tested < 8.3 x 105 WBCs in 95% of units tested pH≥6.2 in at least 90% of units tested . Leukocyte-reduced Apheresis Platelets: At least 3.0 x 1011 platelets in 90% of units tested < 5.0 x 106 WBCs 95% of units tested pH≥6.2 in at least 90% of units tested Methods o Filter: “Fourth-generation” filters remove 99.99% WBCs o Apheresis methods: most apheresis machines have built-in leukoreduction mechanisms o Less efficient methods of reducing WBC content . Washing, deglycerolizing after thawing a frozen unit, centrifugation . These methods do not meet requirement of < 5.0 x 106 WBCs per unit of RBCs/apheresis platelets. Types of leukofiltration/leukoreduction o “Pre-storage” . Done within 24 hours of collection . May use inline filters at time of collection (apheresis) or post collection o “Pre-transfusion” leukoreduction/bedside leukoreduction . Done prior to transfusion . “Bedside” leukoreduction uses gravity-based filters at time of transfusion. Least desirable given variability in practice and absence of proficiency . Alternatively performed by transfusion service prior to issuing Benefits of leukoreduction o Prevention of alloimmunization to donor HLA antigens . Anti-HLA can mediate graft rejection and immune mediated destruction of platelets o Leukoreduced products are indicated for transplant recipients or patients who are likely platelet transfusion dependent o Prevention of febrile non-hemolytic transfusion reactions (FNHTR) . -

Blood Type and Transplantation & A2 Donor to B Recipient
Page 1 of 2 Blood Type and Transplantation Information for Kidney Transplant Patients Does blood type matter in transplantation? Everyone waiting for a transplant has their blood typed. You will have one of four blood types: O, A, B or AB. Your blood type is determined by the antigens that are present on your blood cells. These antigens are A or B. These antigens will be found both in your blood and on your organs. What antigen does each blood type have? Blood type O Blood type A Blood type B Blood type AB have no have A antigens. have B antigens. have both A O antigens. A B AB and B antigens. How does my body react to antigens? Your body will react to antigens that are different than your own by attacking with antibodies. Antibodies are proteins created by your immune system to attack anything that does not belong. Antibodies are the soldiers in your body’s army protecting you from foreign invasions such as viruses. Unfortunately, the antibodies cannot tell the difference between harmful viruses and beneficial transplanted organs. What blood type will my donor be? Transplants can occur between all blood types. However, when the donor’s blood type is different than yours and there are different antigens being transplanted on your new organ, your antibodies will be triggered and attack the transplanted organ. This is called rejection. Because of this, transplants usually happen between a donor and a recipient of the same blood type. This is called an identical transplant. Can I get an organ from a donor that has a different blood type than mine? Yes! If you do not have antibodies in your body against the antigens that come from the donor, your immune system should not attack the transplanted organ. -

Red Blood Cells, Leukocyte Reduced Covenant and AHS Sites
This document applies to all Red Blood Cells, Leukocyte Reduced Covenant and AHS sites. Class: Human blood component, derived from OTHER NAMES: Packed red blood cells, Packed whole blood cells INTRAVENOUS OTHER ROUTES DIRECT IV Intermittent Continuous Infusion Infusion SC IM OTHER Acceptable No Yes No No No No Routes* * Professionals performing these restricted activities have received authorization from their regulatory college and have the knowledge and skill to perform the skill competently. DESCRIPTION OF PRODUCT: Red Blood Cell (RBC) concentrates are prepared from approximately 480mL whole blood usually collected in 70mL of anticoagulant. The unit is plasma reduced by centrifugation, platelet reduced by either centrifugation or filtration and leukocyte reduced by filtration. RBCs are typically resuspended in approximately 110mL of nutrient. Collected from volunteer donors by the Canadian Blood Services (CBS). Donor is screened and blood is tested for: i. Hepatitis B Surface Antigen (HBsAg) ii. Syphilis iii. Antibodies to Hepatitis B core antigen (HBcore), Hepatitis C Virus (HCV), Human T-cell Lymphotropic Virus (HTLV-1 and 2), Human Immune Deficiency Virus (HIV-1 and 2) iv. Nucleic Acid Testing (NAT) for presence of HCV RNA, HIV-1 RNA and West Nile Virus (WNV) RNA . CBS also tests blood for ABO/Rh and clinically significant antibodies. Some donors are tested for CMV status. RBCs do NOT contain any coagulation factors or platelets. AVAILABILITY: . Contact your local transfusion service/laboratory for ABO and Rh types stocked at your site. ABO compatible (not always identical) may be required if the transfusion service cannot provide sufficient quantities of the patient’s blood group (See ABO compatibility chart at: http://www.albertahealthservices.ca/assets/wf/lab/wf-lab-clin-tm-abo-compatability.pdf). -

CCEMS Whole Blood Protocol
PROCEDURES 31 Revised 8/2016 Assessment Approved: Levon Vartanian, MD Hemoglobin Assessment Indications Equipment Needed Trauma or medical patient with suspected blood CLIAwaived photometer, alcohol swab, gauze loss pad, lancet, Capillary Transfer Tube, Test cartridges Procedure 1. Make sure the meter is ready for use. Turn meter on, and when the on-screen display indicates, insert the testing strip all the way in. 2. Cleanse site for capillary puncture using alcohol pad and allow to dry. 3. Lance side of fingertip. Blot away first drop of blood. 4. Press gently once again to get a 2nd drop of blood. 5. Place the tip of the capillary collection tube against the drop of blood. DO NOT squeeze capillary collection tube. 6. Allow capillary collection tube to draw up blood to the level of the black line. 7. Apply blood sample to the specimen area on the test strip. It should fill the entire test area with blood. 8. The meter will then read hemoglobin and hematocrit values. 9. Document findings in PCR. Dispose of microcuvette and lancet into sharps container. Pearls • The meter requires a code chip packaged with each box of testing strips. This chip must be inserted on the left hand side of the meter in slot provided. • Only capillary blood may be tested. Do not use arterial or venous blood. • Samples should be immediately tested. • Hematocrit values will not display if hemoglobin values are outside of the range 12.3–17.5 g/Dl. • Meter will read LO if test result is < 4.5 g/Dl OR if insufficient blood was applied. -

The Johns Hopkins Comprehensive Transplant Center Incompatible Kidney Transplant Programs
The Johns Hopkins Comprehensive Transplant Center Incompatible Kidney Transplant Programs The Johns Hopkins Comprehensive Transplant Center’s Incompatible Kidney Transplantation Program allows many patients previously thought to be “incompatible” to receive the gift of life. The program is comprised of several elements: Blood Type Incompatible Kidney Transplant Program Positive Crossmatch and Sensitized Patient Program Paired Kidney Exhange Program Altruistic Donor Program Blood Type Incompatible Kidney Transplant Program More than one-third of willing live donors are turned down because their blood types are not compatible with the person to whom they wish to donate their kidney. Most of us have natural antibodies against organs from people with different blood types. These antibodies can rapidly destroy a transplanted kidney. The Blood Type Incompatible Transplant Program allows patients to receive a kidney from a live donor who has an incompatible blood type (see fig1.). Patients in this program must be willing to undergo all prescribed treatments before and after the transplant to remove harmful antibodies and decrease the risk of rejection. Figure 1: Blood Type Compatibility Chart Donor Recipient How are harmful antibodies removed? Harmful antibodies are removed with a process called plasmapheresis, a procedure similar to dialysis that removes the plasma portion of the blood where antibodies are located. The number of plasmapheresis treatments required by the recipient before surgery varies depending on the amount of harmful antibodies in their blood. After each plasmapheresis the recipient receives an intravenous infusion of immune globulin to replace antibodies needed to fight infections and help prevent harmful antibodies from returning. Once the antibodies against the donor’s blood type decrease to very low levels, the transplantation can take place. -

Red Blood Cell Transfusions –
Peer Reviewed VETcpd - Internal Medicine Jenny Walton BVM&S MRCVS Jenny qualified from R(D)SVS in Red blood cell transfusions – 1998 - she worked in mixed practice when, what and how to do it! for 4 years before moving into the Red cell transfusions are now a relatively common intervention in veterinary practice field of small in the UK and help in the treatment of many patients. This is largely due to the animal emergency availability of blood products, such as Packed Red Blood Cells (PRBC) , from blood and critical care banks supplying directly to practices. After donation, red cells are separated from with Vets Now for 12 years. Through plasma into a concentrated packed cell form, a nutrient extender is then added to Vets Now, she ran the practical trial them. This allows the red cells to be stored for up to 42 days before being transfused researching canine blood banking in 2005-2006 – launching Pet Blood Bank into patients. DEA 1 blood typing prior to transfusion is essential and cross matching UK (PBB) alongside Wendy Barnett should be performed for second transfusions. Blood products are administered in 2007. She acts as the Veterinary through a filtered giving set and patients monitored closely for transfusion reactions. Supervisor for PBB, her role includes Transfusion reactions are thankfully rare but potentially life threatening. advising practitioners daily on the appropriate use of PBB blood products, Key words: Canine, Packed Red Blood Cells (PRBC), transfusion, blood typing, overseeing the practical and VMD cross matching, transfusion reactions legislative veterinary aspects of blood collection at PBB and leading research on future development opportunities. -

Direct Antibody Test (DAT) Positive
Direct antibody test (DAT) positive Information for patients, parents and guardians As part of your routine pregnancy screening, you However, only a small number of DAT positive have had a direct antibody test (DAT). We’ve babies will develop these problems. Babies given you this factsheet to explain what a DAT who are not DAT positive can still develop involves and what it means if your baby is DAT anaemia and jaundice. A positive DAT simply positive. If you have any further questions, tells us to look out for any signs of anaemia and please speak to a member of your healthcare jaundice. It does not necessarily mean that your team who will be pleased to advise you. baby will need treatment. What is a direct antibody test (DAT)? If we find out that you have rhesus negative During pregnancy, some of the blood between (Rh-) blood during pregnancy, we may give the mother and baby may mix. This mixing of you an injection called anti-D to stop your blood sometimes produces antibodies (proteins body making antibodies against your baby’s that are part of your body’s natural defences) blood. Occasionally this injection causes the which may become a problem for the baby. DAT result to come out positive. Babies who are DAT positive for this reason do not usually As part of routine antenatal (pregnancy) develop anaemia or jaundice. screening blood tests, your midwife will record your blood type (blood group) and check What is anaemia? whether your blood contains any antibodies Anaemia is a very common condition where the that may affect your baby’s red blood cells.