Folate Content and Availability in Malaysian Cooked Foods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LOTUS GASING Menu Mobile
MENU SOUPS S01 VEGETABLE SOUP 5.00 S02 CHICKEN SOUP 6.00 S03 MUTTON SOUP 7.00 APPETIZER S04 VEGETABLE CUTLET 4.00 S05 BITTER GOURD FRY 5.00 S06 ONION BAJJI 5.90 S07 GOBI 65 8.90 S08 MUSHROOM 65 8.90 V01 TAUHU SAMBAL 3.00 V02 VEGETARIAN CHICKEN 7.50 V03 VEGETARIAN MUTTON 8.00 V04 MIXED VEGETABLES ( R / L ) 5.00 / 9.00 V05 BINDI MASALA 14.90 V06 ALOO GOBI MASALA 14.90 V07 CHANNA MASALA 14.90 V08 GREEN PEAS MASALA 16.90 V09 ALOO MUTTER 16.90 V10 PANEER BUTTER MASALA 16.90 V11 PALAK PANEER MASALA 16.90 CHICKEN N01 CHICKEN PARATTAL ( R / M / L ) 8.00 / 15.00 / 30.00 N02 CHICKEN VARUVAL ( R / M / L ) 8.00 / 15.00 / 30.00 N03 CHICKEN 65 ( R / M / L ) 9.00 / 17.00 / 33.00 N04 CHICKEN FRIED / SAMBAL 8.00 NO5 AYAM MASAK MERAH 8.00 N06 CHICKEN RENDANG 8.00 N07 CHICKEN KICAP 8.00 NO8 CHETTINAD PEPPER CHICKEN 16.90 MUTTON N09 MUTTON PARATTAL ( R / M / L )12.00 / 23.00 / 44.00 N10 MUTTON VARUVAL ( R / M / L ) 12.00 / 23.00 / 44.00 UA N11 KODAL CURRY ( GOAT INTESTINE ) 12.00 SUNDAYS PRAWNS N12 PRAWNS SAMBAL ( R / M / L ) 14.00 / 28.00 / 44.00 N13 TIGER PRAWNS 16.00 - 24.00 N14 PRAWNS SPECIAL - SP - SOTONG N15 SOTONG SAMBAL ( R / M / L ) 10.00 / 20.00 / 36.00 N16 SOTONG FRY ( R / M / L ) 10.00 / 20.00 / 36.00 N17 SOTONG SPECIAL - SP - CRAB N18 CRAB CUTLET 8.00 N19 CRAB CURRY / FRY 16.00 - 24.00 FISH N20 FISH CUTLET 6.00 N21 IKAN BULUS 3.00 - 9.00 N22 FRIED FISH 9.00 N23 FISH CURRY 9.00 N24 FISH SAMBAL 9.00 UA N25 DRY FISH CURRY 7.00 SPECIALS ( KARUVADU CURRY ) N26 FISH HEAD CURRY 35.00 - 70.00 N27 FISH SPECIAL - SP- QUAIL N28 QUAIL FRY / SAMBAL 8.00 EGG -

Evaluating Bahulu the Traditional Food: from the Perspective of Consumers
International Food Research Journal 23(6): 2744-2751 (December 2016) Journal homepage: http://www.ifrj.upm.edu.my Evaluating bahulu the traditional food: From the perspective of consumers 1*Abdul Wahid, N. and 2Mudor, H. 1Graduate School of Business, Universiti Sains Malaysia, 11800 Pulau Pinang, Malaysia 2Faculty of Communication Sciences, Prince of Songkla University, Pattani Campus 94000, Thailand Article history Abstract Received: 26 February 2015 Bahulu, a popular traditional food in Malaysia is produced mainly by the Small and Medium Received in revised form: Entrepreneurs (SMEs) who are often micro in size and function as both producers and sellers 22 February 2016 of the food in the marketplace. A problem usually faced by these enterprises is on matching Accepted: 3 March 2016 their bahulu offer with consumers’ preferences that eventually will lead to loss of sales volume, loss of customers and stunted business growth. This study attempts to find out how consumers evaluate bahulu using various aspects of the food product offer. A survey on 671 consumers Keywords from three different states in Malaysia (Penang, Perlis and Kedah) revealed that consumers in general prioritize the product aspect more than on how it is packaged, priced and branded. Traditional snack Overall, halal (label) is the most critical component expected by consumers, followed by Bahulu its color and softness (product), and manufacturer’s address (label). Interestingly, branding, Product attributes packaging and health aspect of the food were amongst the lowest ranked by consumers for this Malaysia product. Some similarities and slight differences in how each consumer group evaluates the bahulu components were also found. -

Food & Entertainment
Food & Entertainment Food Page 35 fe expose Fuego Lights up KL Skyline Level 23A, The Troika is a hotbed of flavours and fun with Troika Fine Dining presenting Cantaloupe, Claret, Strato and now Fuego. Fuego brings scintillating South American flavours to Kuala Lumpur with flamed, grilled and smoked styles dominating a tantalising menu orchestrated by owners Eddie Chew and Christian Bauer. Elevated views of the floodlit Twin Towers provide a spectacular backdrop to this open-fronted outlet. South America is a continent of many national and regional cuisines and Fuego has selected popular styles and dishes from across the land plus some from neighbouring Mexico and Central America. Diners are encouraged to sample various dishes with sharing plates facilitating the process. Ceviche is available in four ways with barramundi, prawn, snapper and salmon prepared in lime. Grilled watermelon with fried halloumi, pickled tomato and raspberry vinegar is one of the more unique entrées. Enjoy desserts such as banana and salted caramel mini magnum ice cream plus a crunchy cookie and banana cream. Wines and spirits such as rums, tequilas and piscos feature on the beverages list. Like all outlets at Troika, bookings are essential with two seatings at 6pm and 8.30pm. www.troikaskydining.com New Japanese Restaurant at Pullman Bangsar Gohan Japanese Restaurant has just opened in a secluded location on the first floor of the Pullman Kuala Lumpur Bangsar to offer what Chef Hoi claims is traditional Japanese cuisine which, according to him, means serving kaiseki (dégustation) or seasonal dishes. The dynamic young chef trained in Singapore under Chef Hirohashi at Kumo Kaiseki Restaurant and then his passion for authentic Japanese food took him to Japan for two years on-the-job training. -

14346 Inai 2020 E.Docx
International Journal of Innovation, Creativity and Change. www.ijicc.net Volume 14, Issue 3, 2020 The Analysis of Food Symbols in the ‘Serarang’ Ritual of the Melanau Likow Community in Dalat, Sarawak Noor Norazila Inaia, Mohamad Maulana Magimanb*, Norhuda Sallehc , d e f Ahmad Nasir Mohd Yusoff & Mangai Tugau , Septian Aji Permana a,e , Ph.D candidate, Department of Social Science, Faculty of Agriculture and Food Science, Universiti Putra Malaysia Bintulu Campus, Sarawak, Malaysia, bSenior Lecturer, Department of Social Science, Faculty of Agriculture and Food Science, Universiti Putra Malaysia Bintulu Campus, Sarawak, Malaysia, cSenior Lecturer, Faculty of Humanities, Arts and Heritage, Universiti Malaysia Sabah, Jalan UMS, 88100 Kota Kinabalu, Sabah, Malaysia, d Senior Lecturer, Department of Government And Civilization Studies, Faculty of Human Ecology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor,Malaysia, Universitas PGRI Yogyakarta, Indonesia, Email: [email protected]. The Serarang ritual is a form of performance performed in the Melanau Likow community to be presented to Ipok. The presentation consists of symbolic elements based on each type of food served. These symbolic elements are involved with the belief and cosmological system that surround the lives of Melanau Likow. The main focus of this research is to analyse the symbol of food in the Serarang ritual from the viewpoint of non-verbal communication. This research used an ethnographic approach and the researchers also participated in the ritual. The data was collected through an in-depth interview with the main informant, Tama Kaul, and with the other ritual practitioners. The research was conducted in Kampung Medong, Dalat Sarawak. The data analysis found that the food that was delivered to Ipok had its own meaning and could be interpreted through shape, colour, and the way it was presented. -

Big-Fish-Premium-Bakery.Pdf
Basic Flavors 8” Round- $40 | 10” Round- $55 tier Cakes 1/2 Sheet - $80 | Full Sheet - $150 BIG FISH Round cakes are 3 layers. $ Half & full sheets are 2 layers. 2- Tiered Cakes ~ 275 Premium Baker y Chocolate or Vanilla ~with chocolate or *serves up to 50 vanilla buttercream Pink Lady~vanilla cake, raspberry jam, vanilla butter cream 3- Tiered Cakes ~ $375 Lemon Berry~lemon cake, raspberry jam, vanilla lemon butter cream *serves up to 100 Carrot Cake~with or without nuts with cream cheese icing $ Sheet Cakes Chocolate Mousse~chocolate cake, 4- Tiered Cakes ~ 475 mocha mousse, chocolate ganache *serves up to 150 Chocolate or vanilla cake w/chocolate or Coconut Cake~ coconut cake, toasted vanilla buttercream w/basic decorations. coconut, coconut butter cream Half - $65 | Full - $120 Red Velvet~ cream chese icing Basic Flavors & Decorations Tres Leches~dulce de leche filling, Flavored Sheet Cakes cinnamon whipped cream topping Chocolate or Vanilla Cake Half - $85 | Full - $160 (not available in half or full sheet) Mousse & Fillings $ Chocolate or Vanilla Extra 15/half sheet Specialty Flavors 8” Round- $45 | 10” Round- $60 Buttercream Flavored cakes are an additional $25/tier. 1/2 Sheet - $100 | Full Sheet - $190 Banana | Coconut | Spiced | Orange | Rum Lemon | Carrot | Red Velvet Sand & sea shells or Half & full sheets are 2 layers. Flavored Buttercream Frostings are an Chocolate Peanut Butter or Nutella~chocolate cake, rose buds with leaves additional $15/tier. peanut butter or Nutella mousse, chocolate ganache Rum Cake~rum soaked butter cake, vanilla mousse Cream Cheese | Coconut | Spiced | Espresso filling, roasted almonds, whipped cream Chocolate Ganache | Orange | Lemon | Caramel Strawberry Shortcake~vanilla cake, Flavored Mousse or Fruit Fillings are whipped cream, fresh strawberries an additional $30/tier. -

Point. Click. Eat. Dineinonline.Com
Point. Click. Eat. dineinonline.com 1 Questions & Answers What is Dine-In? What about drinks? We are a service that allows you to get food deliv- We offer a large variety of sodas in cans or 6-packs. ered from Michiana’s best restaurants! Place your We also offer lemonade, iced tea and bottled water. order with our service and we will make arrange- See back cover for a full drink menu. ments for the restaurant to prepare it and for a driver to deliver it! What hours are you open? How do I order? LUNCH Look through the menus in this book, then call us at (574) 675-9999. A friendly order taker will answer MONDAY - FRIDAY 10:00AM - 2:00PM the phone and guide you through the ordering pro- DINNER cess. Alternatively, visit our website to place your order online: www.dineinonline.com MONDAY - THURSDAY 4:00PM - 10:00PM FRIDAY 4:00PM - 10:30PM How much does it cost? SATURDAY 3:00PM - 10:30PM The restaurants ask that you order a minimum of SUNDAY 3:00PM - 9:00PM $10 worth of food. Restaurant food prices are the same as they would be if you picked up the order What restaurants are available? yourself*. We add a minimal delivery fee, which covers your entire order, no matter how big or Aladdin’s Eatery ..................................................................4 small. Menus and prices are subject to change at Barnaby’s Pizza .................................................................61 any time. To view the most up-to-date menus, visit Beef ‘O’ Brady’s ................................................................14 our website. Between -

Halia Restaurant Ramadhan Buffet 2018 (17/5,20/5,23/5,26/5,29/5,1/6,4/6,7/6,10/6/2018)
HALIA RESTAURANT RAMADHAN BUFFET 2018 (17/5,20/5,23/5,26/5,29/5,1/6,4/6,7/6,10/6/2018) MENU1 Live Stall 1- Appitizer Thai Som Tum Salad, Kerabu Mangga, Sotong Kangkung (Live) Ulam Ulaman Tradisonal (Pegaga, Daun Selom, Ulam Raja, Jantung Pisang, Kacang Botol, Tempe Goreng) Sambal Belacan, Sambal Mangga, Sambal Tempoyak, Cincaluk, Budu, Sambal Gesek Ikan Masin Bulu Ayam, Ikan Masin Sepat dan Ikan Kurau, Ikan Perkasam, Telor Masin Keropok Ikan, Keropok Udang, Keropok Sayur dan Papadhom Live Stall 2 - Mamak Delights Rojak Pasembor with Peanut Sauce & Crackers Live Stall 3 - Soup Aneka Sup Berempah (Bakso Daging, Ayam, Daging, Perut, Tulang Kambing, Tulang Rawan, Ekor, Gear Box) ( Mee Kuning, Bee Hoon, Kuey Teow) Condiments – (Taugeh, Daun Bawang, Daun Sup, Bawang Goreng, Cili Kicap) Roti Benggali Curry Mee with Condiments Bubur - Bubur Lambuk Berherba dan Sambal Main Dishes Ayam Masak Lemak Rebung Stired Fried Beef with Black Pepper Sauce Perut Masak Lemak Cili Padi Ikan Pari Asam Nyonya Prawn with Salted Eggs Sotong Sambal Tumis Petai Stired Fried Pok Choy with Shrimp Paste Nasi Putih Live Stall 4 - Japanese Section Assorted Sushi and Sashimi, Assorted Tempura, Udon / Soba & Sukiyaki Live Stall 5 – Pasta Corner Assorted Pizza (Margarita, Pepperoni, Futi De Mare ) Spaghetti, Penne & Futtuchini with Bolognese, Cabonnara and Tomato Concasse Sauce Live Stall 6 - Sizzler Hot Plate (Assorted Vegetables, Squid, Fish Slice, Clam, Prawn, Mussel, Bamboo Clam) (Sauces: Sweet & Sour, Black Oyster Sauce, Black Pepper & Tom Yam) Live Stall 7 - Steamboat -

Spookilicious Treats!
Each dish is carefully prepared by our Chefs upon order. Enjoy! SALADS LOCAL POTS & DELIGHTS INTERNATIONAL 31 OCTOBER 2020 Fullerton Char Kway Teow Thai Style Chicken Slider 6.30 P.M. TO 10.00 P.M. S$65* per adult; S$33* per child^ Braised Pork Trotter Classic Beer Battered White Fish Add on S$78* for free flow of selected prosecco, SINGAPORE FRUIT ROJAK house rosé, white, red and moscato wines for 2 hours Wok-Fried Oriental Vegetables Crisp Vegetables & Tropical Fruit 24hr Slow-Braised Angus Beef Cheek Add on S$5* for free flow premium coffee and TWG tea Add on S$9* for a glass of Tiger draught Drunken Prawns Gratin Scallops, Garlic Butter, Cheese Add on S$10* for a glass of red or white wine Add on S$15* for a glass of sparkling wine HERITAGE BITES Lala Clams Add on S$20* for a flute of Champagne Singapore Satay SPOOKILICIOUS TREATS! CHEF’S ACTION STATION Eye Ball Cupcakes, Bloody Raspberry ROCK LOBSTER Kurobuta Pork & Crab Meat Ngoh Hiang Pannacotta, Vampire's Teeth Macarons Signature Hainanese Chicken Rice Baked at S$12* per order (half) Salted Egg Chicken Wings Squirmy Worm Cake, Bat Cookie Cupcakes, Roasted Crispy Crackling Pork Belly SEAFOOD ON ICE Oreo Mummy Cookies Tiger Prawns, Norwegian Smoked Salmon, Crispy Spring Rolls Black Mussels Herb-Roasted Angus Ribeye OTHER DESSERTS Fullerton Signature Chocolate Cake, SOUP JAPANESE Tandoori Chicken Mango Pomelo Pannacotta, OMAKASE MAKIMONO Fish Maw Crab Meat Soup Mini Chendol Cake Vegetarian Delight Wild Mushroom Soup Selection of Chef’s Specials Bandung Crème Brûlée, Macaron Maki Roll & Sushi NOODLES OF THE DAY Pulut Hitam Sushi & Sashimi Platter Hand-Pulled Noodles, Mushroom, Chicken PIZZA Bubur Cha Cha Fullerton Pizza of the Day Mini Bowl Rice Dry Hand-Pulled Noodles Classic Margarita Pizza (V) Ice Cream Snack Singapore Laksa PASTA OF THE DAY Sliced Tropical Fruits Cold Angel Hair Pasta . -
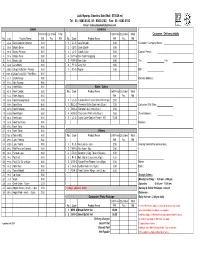
Copy of Order Form Oct 2013 New Price Copy
Lulu Nyonya Goodies Sdn Bhd ( 573034-w) Tel : 03 - 9283 8182, 03 - 9282 6182 Fax : 03 - 9283 3182 Email : [email protected] Sweet Savories Unit Price Qty Order Total Unit Price Qty Order Total Customer / Delivery details No. Code Product Name RM Pcs RM No. Code Product Name RM Pcs RM 1 BS-G Batik Sago wt Coconut 0.80 1 CL-G Cara Berlauk 0.80 Customer / Company Name : 2 BB-G Bingka Beras 0.80 2 CB-G Cucur Badak 0.80 3 BP-G Bingka Pandan 0.80 3 LC-G *Lobak Cake 0.80 Contact Person : 4 BT-G Bingka Telur 0.80 4 CPP-G Mini Pulut Panggang 0.80 5 BU-G Bingka Ubi 0.80 5 POP-G Yam Cake 0.80 Tel : Fax : 6 CM-G Cara Manis 0.80 6 PP-G Curry Puff 1.50 7 CNB-G Crispy Nutty Ball - Peanut 0.80 7 YC-G Popiah 1.50 H/P : 8 CNB1-G Crispy Nutty Ball - Red Bean 0.80 9 CS-G Crystal Sago 0.80 Delivery Address : 10 KLK-G Kole Kacang 0.80 11 KB-G Kueh Bakar 0.80 Bake / Cakes 12 KC-G Kueh Cendol 0.80 No. Code Product Name Unit Price Qty Order Total 13 KJ-G Kueh Jagung 0.80 RM Pcs RM 14 KM-G Kueh Kacang Merah 0.80 1 CC-G *Carrot Cheese Cake Slice(min.30 pc ) 2.50 15 KKR-G Kueh Keria 0.80 2 BCC-G *Premium Butter Cake (min.30 pc ) 1.50 Collection / Del. -

(Cppb) Pada Pembuatan Kerupuk Bandung Di Ukm
library.uns.ac.id digilib.uns.ac.id LAPORAN TUGAS AKHIR KONSEP CARA PRODUKSI PANGAN YANG BAIK (CPPB) PADA PEMBUATAN KERUPUK BANDUNG DI UKM “ELIS BANDUNG” SUKOHARJO, JAWA TENGAH Disusun untuk memenuhi sebagian persyaratan guna memperoleh Derajat Ahli Madya di Fakultas Pertanian Universitas Sebelas Maret Surakarta Disusun oleh: APFIA NIKHO MATICHA H3115011 PROGRAM STUDI DIPLOMA III TEKNOLOGI HASIL PERTANIAN FAKULTAS PERTANIAN UNIVERSITAS SEBELAS MARET SURAKARTA 2019 commit to user i library.uns.ac.id digilib.uns.ac.id commit to user ii library.uns.ac.id digilib.uns.ac.id Halaman Persembahan Dengan segala kerendahan hati dan rasa cinta yang mendalam, tugas akhir ini penulis persembahkan untuk: Allah SWT yang senantiasa melimpahkan rahmat, hidayah dan inayah-Nya, Tuhan Yang Maha Besar dan penguasa semesta alam. Bapak dan Ibu tercinta, atas doa yang tulus dan tiada putus, perjuangan, pengorbanan, kasih saying, serta motivasi dan semangat yang tiada henti. Kakak-kakakku tersayang serta adikku, terimakasih untuk semangat, motivasi dukungan yang telah kalian berikan. Bapak Edhi Nurhartadi dan Ibu Gusti Fauza selaku dosen pembimbing Tugas akhir, atas ilmu, serta arahan dan bimbingan yang diberikan. Sahabat-sahabatku Alfian, Arie, Ardantya, terimakasih selalu memberi semangat dan dukungan dalam suka maupun duka. Teman-teman THP 2015, terimakasih atas kekompakan dan canda tawa, serta semangat yang diberikan dalam suka maupun duka. commit to user iii library.uns.ac.id digilib.uns.ac.id MOTTO Allah SWT berfirman”……….Karena sesungguhnya setelah kesulitan -

Uncle Jim Malaysian Kitchen Commonly Used Ingredients
Uncle Jim Malaysian Kitchen COMMONLY USED INGREDIENTS Belacan Asam Paste Buah Keluak Galangal Lemongrass It is commonly in the It is one the ingredients in (Indonesia Black Nut) It has a light fragrance but is It is a perennial and lemon- form of a pressed brick or the sour dishes. Add water Buah keluak is a black nut not spicy. It is commonly use in scented plant. The outer green cake. Not overly ‘fishy’, to extract tamarind (asam) originating from Indonesia, soup and curries. stalks should be discarded, only a tiny amount of this juice. more famously known for the the bottom part of the whitish paste adds sweetness wonderful aroma in one of the stem may be used. Chef Jim Yong hails from George Town situated in the state of Penang, Malaysia. To to meats, intensity to fish Peranakan’s most famous dish. pursue his interest in food and his love of cooking, Jim moved to Kuala Lumpur to & seafood and a ‘kick’ to vegetables like Kangkung gain wider exposure in the culinary world. He spent time working with experienced “I LOVE GOOD FOOD, Belacan. It makes a chefs and learning new skills from “SIFU”(Master) in the art of cooking. flavourful base for sauces and gravies, adding In addition, to explore the essence of Nyonya cooking, Jim worked with the Nyonya I LOVE THE SMELL OF IT, depth and an intriguing communities in Penang, Melaka, acquiring authentic Nyonya cooking techniques. He Turmeric taste that you can’t quite also learn traditional local cuisine in Terengganu and Kelantan. AND THE KITCHEN ALWAYS decipher. -

60Honourees 10 Awards
A Quarterly Magazine by SMCCI MICA: Number 084/08/2012 • OCTOBER 2016 An Entrepreneurs' Tribute: Weddings On Trend Find out what's hot in the wedding scene. 60 HONOUREES 10 AWARDS MyWork, My Way Get up close and personal with our Working whenever, wherever, and Honourees and the top 16 Finalists. however you want, is now a reality. SPECIAL EDITION: 60TH ANNIVERSARY ISSUE OCTOBER 2016 1 2 BIZVOICE BIZVOICE OCTOBER ISSUE CONTENTS FEATURE FOOD REVIEW by CHEF MEL SME CENTRE @SMCCI 4 An Entrepreneurs’ Tribute: 38 I Am... 29 MyWork, My Way 60 Honourees, 16 Finalists, 10 Awards 32 Expanding Your Company's Horizon CLUSTER HIGHLIGHTS 6 Top Finalists 33 3 STB Grants You Never Knew About 39 SMCCI WE Cluster: Weddings On Trend 12 Award Honourees BUSINESS TIPS 28 A Shelter to call Home 34 Four Rules For Global Leaders To Live By Editorial TEAM EDITORIAL & DESIGN SUPPORT BizVoice is published exclusively for the Singapore Malay Chamber of Commerce & Industry (SMCCI). Fadilah Majid MIRROR SMCCI PR Working [email protected] Have an enterprising story worth sharing? Want to feature Committee, Chairman your products/services to a wider audience? Send you press Writers release, advertising enquiries and all other feedback to Shila Yatiman Muhammad [email protected] or mail them to: SMCCI Executive Director Nurie Arba'ah Nur Khalisah Singapore Malay Chamber of Commerce & Industry (SMCCI) Iylia Mayers Nisa' Tuzzakirah 15 Jalan Pinang Singapore 199147 SMCCI Marketing & Tel: 6297 9296 Communications Executive PRINTER Reproduction in whole or in part is prohibited without prior Syaiful Sam'an NPE PRINT permission from SMCCI.