Mountain Pine Beetle-Attacked Lodgepole Pine for Pulp and Papermaking
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polar Semivolatile Organic Compounds in Biomass-Burning Emissions and Their Chemical Transformations During Aging in an Oxidation flow Reactor
Atmos. Chem. Phys., 20, 8227–8250, 2020 https://doi.org/10.5194/acp-20-8227-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Polar semivolatile organic compounds in biomass-burning emissions and their chemical transformations during aging in an oxidation flow reactor Deep Sengupta, Vera Samburova, Chiranjivi Bhattarai, Adam C. Watts, Hans Moosmüller, and Andrey Y. Khlystov Desert Research Institute, 2215 Raggio Parkway, Reno, NV 89512, USA Correspondence: Vera Samburova ([email protected]) Received: 20 December 2019 – Discussion started: 23 January 2020 Revised: 7 May 2020 – Accepted: 18 May 2020 – Published: 16 July 2020 Abstract. Semivolatile organic compounds (SVOCs) emit- 350 g mol−1 decreased after OFR aging, while abundances ted from open biomass burning (BB) can contribute to chem- of low-MW compounds (e.g., hexanoic acid) increased. This ical and physical properties of atmospheric aerosols and indicated a significant extent of fragmentation reactions in also may cause adverse health effects. The polar fraction of the OFR. Methoxyphenols decreased after OFR aging, while SVOCs is a prominent part of BB organic aerosols, and thus a significant increase (3.7 to 8.6 times) in the abundance of it is important to characterize the chemical composition and dicarboxylic acids emission factors (EFs), especially maleic reactivity of this fraction. In this study, globally and region- acid (10 to 60 times), was observed. EFs for fresh and ratios ally important representative fuels (Alaskan peat, Moscow from fresh-to-aged BB samples reported in this study can be peat, Pskov peat, eucalyptus, Malaysian peat, and Malaysian used to perform source apportionment and predict processes agricultural peat) were burned under controlled conditions occurring during atmospheric transport. -

Resin Acids in Rosin (Colophony) Solder Flux Fume Laboratory Method Using Gas Chromatography
Health and Safety Executive Resin acids in rosin (colophony) solder flux fume Laboratory method using gas chromatography Scope 1 This method describes the measurement of time-weighted average concentrations of rosin (also known as colophony) based solder flux fume collected onto membrane filters with analysis of the resin acid components, after derivatisation, by gas chromatography (GC). Summary 2 A measured volume of air is drawn through a membrane filter mounted in a sampling head close to the breathing zone. The filter is solvent desorbed, the resin acids derivatised and then quantified using GC with a flame ionisation detector (FID). If confirmation of the resin acid components’ identities is required, samples may also be analysed by GC with a mass spectrometer (MS) detector. However, MS is not recommended for quantitative analysis as, unlike an FID, the MS detector gives different response factors for the various resin acids. The use of alternative methods not included in the MDHS series is acceptable provided they can demonstrate the accuracy and reliability appropriate to the application. Recommended sampling 3 For long-term exposures: Maximum sampling time: 8 hours; Sampling rate: 1–2 l-1min; Sampled volume: up to 960 litres. For short-term exposures: Sampling time: 15 mins; Sampling rate: 2 lmin-1; Sampled volume: 30 litres. Prerequisites 4 Users of this method will need to be familiar with the content of MDHS14.1 Safety 5 Users of this method should be familiar with normal laboratory practice and MDHS83/3 carry out a suitable risk assessment. It is the user’s responsibility to establish appropriate health and safety practices and to ensure compliance with regulatory requirements. -

Basics of Kraft Pulping
Lignin Wood is composed of many chemical components, primarily extractives, carbohydrates, and lignin, which are distributed nonuniformly as the result of anatomical structure. Lignin is derived from the Latin term lignum, which means wood.1 Anselme Payen (1838) was the first to recognize the composite nature of wood and referred to a carbon- rich substance as the “encrusting material” which embedded cellulose in the wood. Schulze (1865) later defined this encrusting material as lignin. Lignin has been described as a random, three-dimensional network polymer comprised of variously linked phenylpropane units.2 Lignin is the second most abundant biological material on the planet, exceeded only by cellulose and hemicellulose, and comprises 15-25% of the dry weight of woody plants. This macromolecule plays a vital role in providing mechanical support to bind plant fibers together. Lignin also decreases the permeation of water through the cell walls of the xylem, thereby playing an intricate role in the transport of water and nutrients. Finally, lignin plays an important function in a plant’s natural defense against degradation by impeding penetration of destructive enzymes through the cell wall. Although lignin is necessary to trees, it is undesirable in most chemical papermaking fibers and is removed by pulping and bleaching processes. 1.1.1 Biosynthesis Plant lignins can be broadly divided into three classes: softwood (gymnosperm), hardwood (angiosperm) and grass or annual plant (graminaceous) lignin.3 Three different phenylpropane units, or monolignols, are responsible for lignin biosynthesis.4 Guaiacyl lignin is composed principally of coniferyl alcohol units, while guaiacyl-syringyl lignin contains monomeric units from coniferyl and sinapyl alcohol. -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -
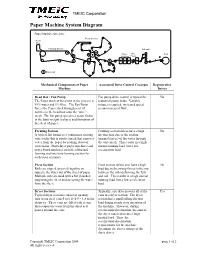
Paper Machine System Diagram
TMEIC Corporation Paper Machine System Diagram Paper Machine One-Line Press Section Head Box Forming Section Calender Dryer Size Reel Sections Press Driven roll Mechanical Components of Paper Associated Drive Control Concepts Regenerative Machine Drives Head Box / Fan Pump Fan pump drive control is typical for No The Paper stock at this point in the process is industrial pump loads. Variable 99% water and 1% fiber. The Fan Pump torque is required, increased speed forces the Paper stock through a set of means increased flow. nozzles in the head box onto the “wire” mesh. The fan pump speed is a major factor in the basis weight (caliper) and formation of the sheet of paper. Forming Section Forming section drives have a high No A typical flat former is a continuous rotating friction load due to the suction wire (today this is plastic) mesh that removes (normal forces) of the water through water from the paper by sucking it out of the wire mesh. This results in a high suspension. Multi-layer paper machines and normal running load, but a low paper board machines include additional acceleration load. forming sections (one forming section for each layer of paper). Press Section Press section drives also have a high No Rolls are nipped (pressed) together to load due to the strong forces in the nip squeeze the water out of the sheet of paper. between the rolls deforming the felts Multiple rolls are used with a felt (blanket) and roll. This results in a high normal supporting the sheet and accepting the water running load, but a low acceleration from the sheet. -
Making Paper from Trees
Making Paper from Trees Forest Service U.S. Department of Agriculture FS-2 MAKING PAPER FROM TREES Paper has been a key factor in the progress of civilization, especially during the past 100 years. Paper is indispensable in our daily life for many purposes. It conveys a fantastic variety and volume of messages and information of all kinds via its use in printing and writing-personal and business letters, newspapers, pamphlets, posters, magazines, mail order catalogs, telephone directories, comic books, school books, novels, etc. It is difficult to imagine the modern world without paper. Paper is used to wrap packages. It is also used to make containers for shipping goods ranging from food and drugs to clothing and machinery. We use it as wrappers or containers for milk, ice cream, bread, butter, meat, fruits, cereals, vegetables, potato chips, and candy; to carry our food and department store purchases home in; for paper towels, cellophane, paper handkerchiefs and sanitary tissues; for our notebooks, coloring books, blotting paper, memo pads, holiday greeting and other “special occasion’’ cards, playing cards, library index cards; for the toy hats, crepe paper decorations, paper napkins, paper cups, plates, spoons, and forks for our parties. Paper is used in building our homes and schools-in the form of roofing paper, and as paperboard- heavy, compressed product made from wood pulp-which is used for walls and partitions, and in such products as furniture. Paper is also used in linerboard, “cardboard,” and similar containers. Wood pulp is the principal fibrous raw material from which paper is made, and over half of the wood cut in this country winds up in some form of paper products. -

Make Your Own Paper Students Investigate the Papermaking Process by Trying It Themselves
Make Your Own Paper Students investigate the papermaking process by trying it themselves. Students are Activity thrilled to find that they can make paper and that their product is practical, as well as beautiful. See the PLT website, www.plt.org, to watch a video of the paper-making 51 process used in this activity. Levels OBJECTIVE ASSESSMENT OPPORTUNITIES Grades 1-8 Subjects n Students will describe the steps of the paper- n Ask younger students to write the directions Science, Social Studies, making process and identify the elements and for making paper on the piece of recycled paper Language Arts, Visual Arts outputs of the process. that they made. n Concepts Have students use concept mapping, graphics software, or write a script for a n By reducing waste and recy- video that explains the papermaking process. cling materials, individuals and societies can extend the value and utility of resources and can promote environ- mental quality. (2.7) The process begins when trees, grown BACKGROUND Skills especially for papermaking, are harvested Observing, Organizing and transported to a paper mill. At the Information, Comparing and Paper is a simple material. It is essentially mill, large machines strip away bark and Contrasting a mat held together by a fiber’s rough- shred the logs into millions of chips the ness, and can be made from almost any size of breakfast cereal. The wood chips fibrous material such as cotton, hemp, travel on conveyors to gigantic “pulp Higher Order Thinking, Paired/ flax, wood or recycled paper. And yet, this Cooperative Learning, Realia/ cookers,” where chemicals and steam are Hands-on Learning simple product has a tremendous effect added. -

Pulp & Paper News
Pulp & Paper 21 Perspectives in Liquid Process Analytics News INGOLD Leading Process Analytics Streamline Your Processes with the New iSense Software Intelligent Sensor Management (ISM®) technology is helping pulp and paper mills the world over to increase process reliability, reduce sensor lifecycle costs, and simplify sensor handling. With the new iSense software for ISM sensors, realizing the benefits of digital sensor technology is easier than ever. Significant benefits lytical equipment the more efficient will Analytical measurements are going digi- be your processes. tal. The advantages offered by the latest, cutting-edge in-line sensors and transmit- iSense, the accompanying software for ters, such as greater process quality and ISM, streamlines all your sensor activities. yield, reduced sensor maintenance, and It provides highly valuable features such simplified sensor handling, are hard to as sensor calibration away from the pro- ignore. cess, electronic documentation, instant evaluation of a sensorʼs “health”, and ISM, METTLER TOLEDOʼs digital sensor predictive information on when mainte- technology, has transformed the way ana- nance will be required. The latest version lytical sensors are handled and main- of iSense enables seamless management tained from first installation to end of life. of ISM sensors and delivers exceptional ISM offers a level of performance and usability. convenience that is not available with other systems. It is easier with iSense Spending hours learning new software is Convenience is the key a costly use of operator time, so we have Whether in production or in the lab, the made iSense extremely intuitive to greater the convenience provided by ana- operate. For a new sensor, just connect the Blue- tooth® communicator supplied with the software. -

United States Patent \ .J Patented Oct
2,907,738 United States Patent \ .j Patented Oct. 3, 1959 1 2 dibasic acids would also give polymerization in which the natural resin acid ester becomes an intimate part ‘ 2,907,738 of the ?nal polymer chemical composition. Such esteri ‘MIXED RESIN ACID ESTERS OF 4,4-BIS(47 ?cation reactions involving the phenolic hydroxyl groups HYDROXYARYL) PENTANOIC ACID 01 would conveniently be carried out by heating with a mix ture of the dibasic acid and acetic anhydride. Sylvan 0. Greenlee, Racine, Wis., assignor to The hydroxyaryl—substituted aliphatic acids contem~ S. C. Johnson & Son, Inc., Racine, Wis. plated for use in preparing the desired resinous poly No Drawing. Application June 30,1955 hydric phenols have two hydroxyphenyl groups attached ‘ Serial No.‘ 519,279’ 10 to a single carbon atom. The preparation of these sub~ stituted acids may be most conveniently carried out by 9 Claims. (Cl. 260-24)‘ condensing a keto-acid with the desired phenol. Expe rience in the preparation of bisphenols and related com pounds indicates that the carbonyl group of the keto This invention relates to new compositions which are 15 acid must be located next to a terminal carbon atom in ‘mixed esters of‘polyhydric alcohols, natural resin,acids, order to obtain satisfactory yields. A terminal carbon and hydroxyaryl-substituted aliphatic acids. atom as used herein refers to primary carbon atoms An object of this invention is to produce new com other ‘than the carboxyl carbon atom of the keto-acid. positions from natural resin acids, polyhydric alcohols Prior applications, Serial Nos. 464,607 and 489,300, and hydroxyaryl-substituted aliphatic acids which are 20 ?led October 25, 1954, and February 18, 1955, respec~ valuable as intermediates in the production of other more tively, disclose a number of illustrative compounds suit complex compositions. -

Construction Health and Safety Manual: Pulp and Paper Mills
PULP AND PAPER MILLS 33 PULP AND PAPER MILLS The two common forms of chemical pulping are 1) the dominant “alkaline” or “kraft” process, and Processes 2) the “acid pulping” or “sulphite” process. Acid pulping has generally declined but is still in use. The A number of processes, grouped by type as mechanical, digester liquor is a solution of sulphurous acid, H SO , chemical, and semi-chemical (or hybrid), are used in 2 3 mixed with lime (CaO) or other base (magnesium, the preparation of wood pulp. In 1990 (according to sodium, or ammonium) to form bisulphites. Lockwood’s Directory) the distribution of pulp mills in Ontario and Quebec was as follows: Mechanical processes produce the highest yield from the wood, but have high energy demands. Mechanical pulping Process Type generally incorporates thermal or chemical pre-softening Chemical Processes Semi-chemical Mechanical Total of the wood chips, resulting in lower energy requirements. Kraft Sulphite Some chemical processes include mechanical features. Ontario 94 2 15 30 The division is not distinct and is generally based on Quebec 10 8241 61 efficiency of production from dry wood. Figure 22.1: Number of pulp mills by type in Ontario and Quebec Figure 22.2 provides a flow diagram for a semi-chemical pulp mill. In chemical pulping, the wood chips are cooked, using heat and a chemical solution that depends on the type of Of the chemical processes , alkaline pulping – the kraft process being used. The lignin binder, a natural glue that or sulphite process – is the most common and is shown in holds the wood cells (fibres) together, is dissolved. -

Paper History
Volume 14, Year 2010, Issue 1 PAPER HISTORY Journal of the International Association of Paper Historians Zeitschrift der Internationalen Arbeitsgemeinschaft der Papierhistoriker Revue de l’Association Internationale des Historiens du Papier ISSN 0250-8338 www.paperhistory.org PAPER HISTORY, Volume 14, Year 2010, Issue 1 International Association of Paper Historians Contents / Inhalt / Contenu Internationale Arbeitsgemeinschaft der Papierhistoriker Letter from the President May 2010 3 Lettre de la présidente de l’IPH – may 2010 3 Association Internationale des Historiens du Papier Brief der IPH-Präsidentin, Mai 2010 4 Important plan for the reco-very of several papermills on the Amalfi Coast 5 Pulp and Paper on Stamps 8 Le congrès à Angoulême 16 IPH Assemblée générale, Angoulême (France), 9 octobre, 2010 17 Information from delegates 20 General information 23 Orbituaries 24 Guidelines for authors 26 Editor Anna-Grethe Rischel Complete your paper historical library now! Denmark Ergänzen Sie jetzt Ihre papierhistorische Co-editors IPH-Delegates Bibliothek! Maria Del Carmen Hidalgo Brinquis Completez aujourd’hui votre bibliothèque de Spain l’Histoire du papier! 27 Dr. Claire Bustarret France Prof. Dr. Alan Crocker United Kingdom Dr. Józef Dąbrowski Poland Jos De Gelas Belgium Deadline for contributions each year 15. March and 15. September Elaine Koretsky USA Paola Munafò Italy President Anna-Grethe Rischel Dr. Henk J. Porck Präsident Stenhøjgaardsvej 57 The Netherlands President DK - 3460 Birkerød Prof. Dr. Gottfried Schweizer Denmark Austria tel + 45 45 816803 [email protected] Prof. Dr. Tomas Stohr Venezuela Secretary Dr. Sabine Schachtner Göran Wohlfahrt Sekretariat LVR-Industriemuseum Sweden Secrétaire Papiermühle Alte Dombach Lay-out Karen Borchersen D- 51465 Bergisch Gladbach The School of Conservation Germany Esplanaden 34 tel + 49 2202 936880 DK – 1263 Copenhagen K [email protected] Denmark [email protected] Treasurer Alphonse Radermecker Printer Prinfo Paritas Printcenter Kassier Hochstr. -

Determination of Terpenoid Content in Pine by Organic Solvent Extraction and Fast-Gc Analysis
ORIGINAL RESEARCH published: 25 January 2016 doi: 10.3389/fenrg.2016.00002 Determination of Terpenoid Content in Pine by Organic Solvent Extraction and Fast-GC Analysis Anne E. Harman-Ware1* , Robert Sykes1 , Gary F. Peter2 and Mark Davis1 1 National Bioenergy Center, National Renewable Energy Laboratory, Golden, CO, USA, 2 School of Forest Resources and Conservation, University of Florida, Gainesville, FL, USA Terpenoids, naturally occurring compounds derived from isoprene units present in pine oleoresin, are a valuable source of chemicals used in solvents, fragrances, flavors, and have shown potential use as a biofuel. This paper describes a method to extract and analyze the terpenoids present in loblolly pine saplings and pine lighter wood. Various extraction solvents were tested over different times and temperatures. Samples were analyzed by pyrolysis-molecular beam mass spectrometry before and after extractions to monitor the extraction efficiency. The pyrolysis studies indicated that the optimal extraction method used a 1:1 hexane/acetone solvent system at 22°C for 1 h. Extracts from the hexane/acetone experiments were analyzed using a low thermal mass modular accelerated column heater for fast-GC/FID analysis. The most abundant terpenoids from Edited by: the pine samples were quantified, using standard curves, and included the monoter- Subba Rao Chaganti, University of Windsor, Canada penes, α- and β-pinene, camphene, and δ-carene. Sesquiterpenes analyzed included Reviewed by: caryophyllene, humulene, and α-bisabolene. Diterpenoid resin acids were quantified in Yu-Shen Cheng, derivatized extractions, including pimaric, isopimaric, levopimaric, palustric, dehydroabi- National Yunlin University of Science and Technology, Taiwan etic, abietic, and neoabietic acids.