A Critique of "Bromeliales, Related Monocots, and Resolution of Relationships Among Bromeliaceae Subfamilies" Source: Systematic Botany, Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I Scope and Importance of Taxonomy. Classification of Angiosperms- Bentham and Hooker System & Cronquist
Syllabus: 2020-2021 Unit – I Scope and importance of Taxonomy. Classification of Angiosperms- Bentham and Hooker system & Cronquist. Flora, revision and Monographs. Botanical nomenclature (ICBN), Taxonomic hierarchy, typification, principles of priority, publication, Keys and their types, Preparation and role of Herbarium. Importance of Botanical gardens. PLANT KINGDOM Amongst plants nearly 15,000 species belong to Mosses and Liverworts, 12,700 Ferns and their allies, 1,079 Gymnosperms and 295,383 Angiosperms (belonging to about 485 families and 13,372 genera), considered to be the most recent and vigorous group of plants that have occurred on earth. Angiosperms occupy the majority of the terrestrial space on earth, and are the major components of the world‘s vegetation. Brazil (First) and Colombia (second), both located in the tropics considered to be countries with the most diverse angiosperms floras China (Third) even though the main part of her land is not located in the tropics, the number of angiosperms still occupies the third place in the world. In INDIA there are about 18042 species of flowering plants approximately 320 families, 40 genera and 30,000 species. IUCN Red list Categories: EX –Extinct; EW- Extinct in the Wild-Threatened; CR -Critically Endangered; VU- Vulnerable Angiosperm (Flowering Plants) SPECIES RICHNESS AROUND THE WORLD PLANT CLASSIFICATION Historia Plantarum - the earliest surviving treatise on plants in which Theophrastus listed the names of over 500 plant species. Artificial system of Classification Theophrastus attempted common groupings of folklore combined with growth form such as ( Tree Shrub; Undershrub); or Herb. Or (Annual and Biennials plants) or (Cyme and Raceme inflorescences) or (Archichlamydeae and Meta chlamydeae) or (Upper or Lower ovarian ). -

Arthur Monrad Johnson Colletion of Botanical Drawings
http://oac.cdlib.org/findaid/ark:/13030/kt7489r5rb No online items Arthur Monrad Johnson colletion of botanical drawings 1914-1941 Processed by Pat L. Walter. Louise M. Darling Biomedical Library History and Special Collections Division History and Special Collections Division UCLA 12-077 Center for Health Sciences Box 951798 Los Angeles, CA 90095-1798 Phone: 310/825-6940 Fax: 310/825-0465 Email: [email protected] URL: http://www.library.ucla.edu/libraries/biomed/his/ ©2008 The Regents of the University of California. All rights reserved. Arthur Monrad Johnson colletion 48 1 of botanical drawings 1914-1941 Descriptive Summary Title: Arthur Monrad Johnson colletion of botanical drawings, Date (inclusive): 1914-1941 Collection number: 48 Creator: Johnson, Arthur Monrad 1878-1943 Extent: 3 boxes (2.5 linear feet) Repository: University of California, Los Angeles. Library. Louise M. Darling Biomedical Library History and Special Collections Division Los Angeles, California 90095-1490 Abstract: Approximately 1000 botanical drawings, most in pen and black ink on paper, of the structural parts of angiosperms and some gymnosperms, by Arthur Monrad Johnson. Many of the illustrations have been published in the author's scientific publications, such as his "Taxonomy of the Flowering Plants" and articles on the genus Saxifraga. Dr. Johnson was both a respected botanist and an accomplished artist beyond his botanical subjects. Physical location: Collection stored off-site (Southern Regional Library Facility): Advance notice required for access. Language of Material: Collection materials in English Preferred Citation [Identification of item], Arthur Monrad Johnson colletion of botanical drawings (Manuscript collection 48). Louise M. Darling Biomedical Library History and Special Collections Division, University of California, Los Angeles. -

The Fossil Record of Angiosperm Families in Relation to Baraminology
The Proceedings of the International Conference on Creationism Volume 7 Article 31 2013 The Fossil Record of Angiosperm Families in Relation to Baraminology Roger W. Sanders Bryan College Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Sanders, Roger W. (2013) "The Fossil Record of Angiosperm Families in Relation to Baraminology," The Proceedings of the International Conference on Creationism: Vol. 7 , Article 31. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol7/iss1/31 Proceedings of the Seventh International Conference on Creationism. Pittsburgh, PA: Creation Science Fellowship THE FOSSIL RECORD OF ANGIOSPERM FAMILIES IN RELATION TO BARAMINOLOGY Roger W. Sanders, Ph.D., Bryan College #7802, 721 Bryan Drive, Dayton, TN 37321 USA KEYWORDS: Angiosperms, flowering plants, fossils, baramins, Flood, post-Flood continuity criterion, continuous fossil record ABSTRACT To help estimate the number and boundaries of created kinds (i.e., baramins) of flowering plants, the fossil record has been analyzed. To designate the status of baramin, a criterion is applied that tests whether some but not all of a group’s hierarchically immediate subgroups have a fossil record back to the Flood (accepted here as near the Cretaceous-Paleogene boundary). -

MOLECULAR EVOLUTION, ADAPTIVE RADIATION, and GEOGRAPHIC DIVERSIFICATION in the AMPHIATLANTIC FAMILY RAPATEACEAE: EVIDENCE from Ndhf SEQUENCES and MORPHOLOGY
Evolution, 54(6), 2000, pp. 1915±1937 MOLECULAR EVOLUTION, ADAPTIVE RADIATION, AND GEOGRAPHIC DIVERSIFICATION IN THE AMPHIATLANTIC FAMILY RAPATEACEAE: EVIDENCE FROM ndhF SEQUENCES AND MORPHOLOGY T. J. GIVNISH,1,2 T. M. EVANS,3 M. L. ZJHRA,4 T. B. PATTERSON,1 P. E. BERRY,1 AND K. J. SYTSMA1 1Department of Botany, University of Wisconsin, Madison, Wisconsin 53706 2E-mail: [email protected] 3Department of Biology, Hope College, Holland, Michigan 49423 4Department of Biology, University of Arizona, Tucson, Arizona 85721 Abstract. Rapateaceae (16 genera, ;100 species) is largely restricted to the tepuis and sandplains of the Guayana Shield in northern South America, with Maschalocephalus endemic to West Africa. The family has undergone extensive radiation in ¯ower form, leaf shape, habit, and habitat. To analyze the evolution of these distributions and traits, we derived a molecular phylogeny for representatives of 14 genera, based on sequence variation in the chloroplast-encoded ndhF gene. The lowland subfamily Rapateoideae is paraphyletic and includes the largely montane subfamily Saxo- fridericioideae as a monophyletic subset. Overall, the morphological/anatomical data differ signi®cantly from ndhF sequences in phylogenetic structure, but show a high degree of concordance with the molecular tree in three of four tribes. Branch lengths are consistent with the operation of a molecular clock. Maschalocephalus diverges only slightly from other Monotremae: it is the product of relatively recent, long-distance dispersal, not continental driftÐonly its habitat atop rifted, nutrient-poor sandstones is vicariant. The family appears to have originated approximately 65 Mya in inundated lowlands of the Guayana Shield, followed by: (1) wide geographic spread of lowland taxa along riverine corridors; (2) colonization of Amazonian white-sand savannas in the western Shield; (3) invasion of tepui habitats with frequent speciation, evolution of narrow endemism, and origin of hummingbird pollination in the western Shield; and (4) reinvasion of lowland white-sand savannas. -

Phylogenetics of Seed Plants: an Analysis of Nucleotide Sequences from the Plastid Gene Rbcl James F
Boise State University ScholarWorks Biology Faculty Publications and Presentations Department of Biological Sciences 1-1-1993 Phylogenetics of Seed Plants: An Analysis of Nucleotide Sequences from the Plastid Gene rbcL James F. Smith Boise State University This document was originally published by Biodiversity Heritage Library in Annals of the Missouri Botanical Garden. Copyright restrictions may apply. Image courtesy of Biodiversity Heritage Library. http://biodiversitylibrary.org/page/553016 See article for full list of authors. PHYLOGENETICS OF SEED Mark TV Chase,' Douglas E. Soltis,' PLANTS: AN ANALYSIS OF R ichard C. Olmstead;' David Morgan,J Donald H Les,' Brent D. Mishler," NUCLEOTIDE SEQUENCES Melvin H, Duvall,' Hobert A. Price,' FROM THE PLASTID Harold G. Hills,' Yin· Long Qiu,' Kathleen A. Kron,' Jeffrey H. Hell.ig,' GENE rb ell Elena Cont.':, 10 Jeffrey D. Palmer, 1l James R. ft1an/wrt,9 Kenneth}. Sylsma, 1O Helen J . Michaels," W. John Kress, " Kenneth C. Karol, 1O W. Dennis Clark, 13 lvlikael J-I edren,u Brandon S. Gaut,? Robert K. J ansen, l ~ Ki.Joong Kim ,'5 Charles F. Jf/impee,5 James F. Smilh,I2 Glenn N. Furnier,lo Steven 1-1. Strauss,l? Qiu- Yun Xian g,3 Gregory 1.1. Plunkeu,J Pamela S. Soltis ,:" Susan M. Swensen, ]!J Stephen /, ', Williams," Paul A. Gadek,'" Christopher }. Quinn,20 Luis E. /:.guiarle,7 Edward Golenberg," Gerald 1-1. Learn, jr.,7 Sean W. Graharn,22 Spencer C. H. Barreu," Selvadurai Dayanandan,23 and Victor A. Albert' AIJSTRACf We present the results of two exploratory parsimony analyses of DNA sequences from 475 and 499 species of seed plants. -

DDC) Stemming from the Adoption of the APG (Angiosperm Phylogeny Group) III Classification As the Basis for the DDC’S Treatment of Flowering Plants
This PDF documents proposed changes throughout the Dewey Decimal Classification (DDC) stemming from the adoption of the APG (Angiosperm Phylogeny Group) III classification as the basis for the DDC’s treatment of flowering plants. We request comment from any interested party, to be sent to Rebecca Green ([email protected]) by 31 January 2016. Please include “Angiosperm review comments” in your subject line. -------------------------------------------------------------- Why is the DDC adopting a new basis for classifying angiosperms (flowering plants)? During the latter half of the 20th century, biological classification turned from establishing taxa predominantly on the basis of morphological similarities to establishing taxa predominantly on the basis of shared ancestry / shared derived characters, with biological taxonomies mirroring evolutionary relationships. Phylogenetic analysis typically underlies modern evolutionary classifications, but has resulted in the development of many competing classifications. Within the domain of flowering plants, different classification systems have been favored in different countries. The Angiosperm Phylogeny Group, a global consortium of botanists, has addressed this issue by developing a “consensus” classification that is monophyletic (i.e., its taxa include all but only the descendants of a common ancestor). Now in its third version, the APG III classification is considered relatively stable and useful for both research and practice (e.g., for organizing plants in herbaria). The development for flowering plants presented here is the culmination of DDC editorial work over a span of several years. An early version revised 583–584 to make the schedule compatible with the APG III classification, while trying to minimize relocations and using see references to establish the APG III logical hierarchy. -
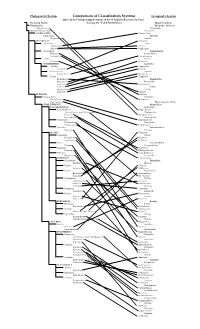
Comparison of Cronquist and Phylogeneticalt
Phylogenetic System Comparison of Classification Systems Cronquist's System Lines Show Corresponding Positions of the 60 Families Reviewed in Class Flowring Plants Biology 211 - Fall 2002 (Phillips) Magnoliophyta Nymphaeales Liliopsida - Monocots Nymphaeaceae Alismatidae (unnamed major clade) Alismatales MAGNOLIIDS Alismataceae Magnoliales Arecidae Magnoliaceae Arecales MONOCOTS Arecaceae Alismatales Arales Alismataceae Araceae Araceae (incl. Lemnaceae) Lemnaceae Asparagales Commelinidae Iridaceae Commelinales Orchidaceae Commelinaceae Liliales Juncales Liliaceae Juncaceae COMMELINIDS Cyperales Arecales Cyperaceae Arecaceae Poaceae Commelinales Typhales Commelinaceae Sparganiaceae Poales Typhaceae Bromeliaceae Zingiberidae Cyperaceae Bromeliales Juncaceae Bromeliaceae Poaceae Lilidae Sparganiaceae Liliales Typhaceae Liliaceae EUDICOTS Iridaceae Ranunculales Orchidales Ranunculaceae Orchidaceae Papaveraceae Magnoliopsida - Dicots CORE EUDICOTS Magnoliidae Caryophyllid Clade Magnoliales Caryophyllales Magnoliaceae Amaranthaceae (incl. Chenopodiaceae) Nymphaeales Cactaceae Nymphaeaceae Caryophyllaceae Ranunculales Polygonales Ranunculaceae Droseraceae Papaverales Polygonaceae Papaveraceae Santalales Hamamelididae Viscaceae Urticales ROSIDS Ulmaceae Saxifragales Fagales Crassulaceae Fagaceae Saxifragaceae Betulaceae Vitales Caryophyllidae Vitaceae Caryophyllales Geraniales Cactaceae Geraniaceae Chenopodiaceae Myrtales Caryophyllaceae Onagraceae Polygonales EUROSIDS I Polygonaceae Cucurbitales Dilleniidae Cucurbitaceae Malvales Fabales Tiliaceae -
A Listing of the Flora and Fauna of Saint Helena Island, South Carolina with Emphasis on Historic Penn Center
Journal of the South Carolina Academy of Science 4(1):33-47 Fall 2007 A LISTING OF THE FLORA AND FAUNA OF SAINT HELENA ISLAND, SOUTH CAROLINA WITH EMPHASIS ON HISTORIC PENN CENTER Steven E. Fields1, Harriet Speer, Joshua S. Castleberry, Michael W. Hook, Rebecca Hunsucker, and David M.Lambert School of the Environment, University of South Carolina, 702G Byrnes Bldg., Columbia, SC 29208 Alternate address: Culture and Heritage Museums, 4621 Mount Gallant Rd, Rock Hill, SC 29732 [email protected] ABSTRACT We present baseline occurrence data for at least 306 taxa of plants and animals in the vicinity of Historic Penn Center on St. Helena Island, South Carolina. It is hoped that this list will be appended by future surveyors and can serve as an aid in the conservation of species and the preservation of the natural, as well as the cultural history of the site. INTRODUCTION The University of South Carolina course, Natural History of the South Carolina Low Country, took place May 7-12, 2006 at the Penn Center, on Saint Helena Island, one of the South Carolina Sea Islands, located off the coast of Beaufort (Fig. 1). The area is classified as Sea Island/Coastal Marsh in the Southern Coastal Plain within the Level III and IV Ecoregions of South Carolina (Griffith et al. 2002). Figure 1. Location of St. Helena Island, South Carolina The Penn Center is situated on 50 acres in the heart of Gullah country. The site is rich in natural history, but also in cultural history. From its origin in 1862 as the Penn School, it became an experimental program to educate Sea Island slaves freed at the beginning of the Civil War. -
Systematics and Evolution of the Genus Deuterocohnia Mez (Bromeliaceae)
Systematics and evolution of the genus Deuterocohnia Mez (Bromeliaceae) Dissertation zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) Vorgelegt im Fachbereich Naturwissenschaften der Universität Kassel von Dipl.-Biol. Nicole Schütz Kassel, 2011 Vom Fachbereich Naturwissenschaften der Universität Kassel als Dissertation angenommen. Dekan: Prof. Dr. Friedrich W. Herberg Gutachter: Prof. Dr. Kurt Weising und Prof. Dr. Georg Zizka Prüfungskommission: Prof. Dr. Pierre Ibisch, Prof. Dr. Rüdiger Wagner, Prof. Dr. Kurt Weising, Prof. Dr. Georg Zizka Datum der Disputation: 21.02. 2012 Content I Content 1 INTRODUCTION .....................................................................................................1 1.1 Biology and systematics of the Bromeliaceae Juss......................................................1 1.2 The genus Deuterocohnia Mez................................................................................... 5 1.3 Scope of the present study .......................................................................................... 9 2 MATERIAL AND METHODS................................................................................. 11 2.1 Sources of plant material............................................................................................ 11 2.1.1 Living plant material ....................................................................................................... 11 2.1.2 Herbarium specimens.................................................................................................... -
Does the Monocot Mode of Leaf Development Characterize All Monocots? Geeta Bharathan University of California, Davis
Aliso: A Journal of Systematic and Evolutionary Botany Volume 14 | Issue 4 Article 6 1995 Does the Monocot Mode of Leaf Development Characterize all Monocots? Geeta Bharathan University of California, Davis Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Bharathan, Geeta (1995) "Does the Monocot Mode of Leaf Development Characterize all Monocots?," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 14: Iss. 4, Article 6. Available at: http://scholarship.claremont.edu/aliso/vol14/iss4/6 Aliso, 14(4}, pp. 271-279 © 1996, by The Rancho Santa Ana Botanic Garden, Claremont, CA 91711-3157 DOES THE MONOCOT MODE OF LEAF DEVELOPMENT CHARACTERIZE ALL MONOCOTS? GEETA BHARATHAN Agronomy and Range Science University of California Davis, California 95616 FAX: 916/152-4361 e-mail: grbharathan@ ucdavis. edu ABSTRACT Patterns of early leaf development in monocots are analyzed in a phylogenetic context. Recent developmental and phylogenetic studies enable this reevaluation of the leaf base model of the devel oping monocot leaf. Two questions are addressed: a) is the presence of the Vorlauferspitze (fore-runner tip) invariably correlated with development of the lamina from the lower leaf zone? and b) was the ancestral monocot characterized by the leaf base mode of development? Scanning electron microscopic observations are made of young primorida using the mold and cast method. These data are combined with data from the literature and examined in a phylogenetic context using parsimony analysis. The results suggest that in some taxa the Vorlauferspitze may be associated with a lamina that is derived from the upper leaf zone, and that the ancestral monocot may not have been characterized by the leaf base mode of development. -

Classification, Evolution, and Phylogeny of the Families of Monocotyledons
SMITHSONIAN CONTRIBUTIONS TO BOTANY NUMBER 71 Classification, Evolution, and Phylogeny of the Families of Monocotyledons Aaron Goldberg SMITHSONUN INSTITUTION PRESS Washington, D.C. 1989 ABSTRACT Goldberg, Aaron. Classification, Evolution, and Phylogeny of the Families of Monocotyle- dons. Smithsonian Contributions to Botany, number 71, 74 pages, 41 figures, 2 tables, 1 diagram, 1989.-To some extent classification is subjective. Taxonomists differ in the relative importance they ascribe to particular characters and in the degree of difference between related taxa they deem sufficient to constitute family or ordinal rank. About 250 monocot family names have been published. Those who have attempted an overview of the system at the family level and above in the last quarter century recognize between 45 and 103 monocot families in 14 to 38 orders. I accept 57 families in 18 orders. In Table 1 I give my ordinal allocation of the families and that of 11 recent authors to indicate where there is agreement and where there are differences to be resolved. I have constructed a dendrogram to suggest relationships and degree of advancement of the orders. I have written concise, uniform descriptions of all the families of monocots emphasizing those characters that show trends between families or occur in more than one family. Each family is illustrated by analytical drawings of the flower, fruit, seed, and usually inflorescence. Several species are usually used to show the range of major variation within families and trends toward related families. Monocots and dicots have existed concurrently for most of their history, have been subjected to many of the same ecological pressures, and consequently show similar evolutionary trends. -

Flora of the Guianas Project
Leiden, October 2012 The Flora of the Guianas is a co-operative programme of: Botanischer Garten und Botanisches Museum Berlin-Dahlem, Berlin; Institut de Recherche pour le Développement, IRD, Centre de Cayenne, Cayenne; Department of Biology, University of Guyana, Georgetown; Herbarium, Royal Botanic Gardens, Kew; New York Botanical Garden, New York; Nationaal Herbarium Suriname, Paramaribo; Muséum National d’Histoire Naturelle, Paris; Nationaal Herbarium Nederland, Utrecht University branch, Utrecht, and Department of Botany, Smithsonian Institution, Washington, D.C. For further information see the website: http://www.nationaalherbarium.nl/FoGWebsite/index.html Published on June 2014 Flora of the Guianas Newsletter no 18. Nationaal Herbarium Nederland, Naturalis Biodiversity Center, Einsteiweg 2, 2333 CC, Leiden, The Netherlands 2 CONTENTS In Memorian M.F.Prévost, J. Florschutz 1. MEETING PROGRAM ...................................................................................................... 6 2. MINUTES OF THE ADVISORY BOARD MEETING .............................................................. 7 2.1. Opening and report on previous meeting in Washigton .............................................. 7 2.2. Board personnel changes ............................................................................................ 7 2.3. Memorandum of Understanding ................................................................................. 7 2.4. Report by the executive editor ...................................................................................