Arthur Monrad Johnson Colletion of Botanical Drawings
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

I Scope and Importance of Taxonomy. Classification of Angiosperms- Bentham and Hooker System & Cronquist
Syllabus: 2020-2021 Unit – I Scope and importance of Taxonomy. Classification of Angiosperms- Bentham and Hooker system & Cronquist. Flora, revision and Monographs. Botanical nomenclature (ICBN), Taxonomic hierarchy, typification, principles of priority, publication, Keys and their types, Preparation and role of Herbarium. Importance of Botanical gardens. PLANT KINGDOM Amongst plants nearly 15,000 species belong to Mosses and Liverworts, 12,700 Ferns and their allies, 1,079 Gymnosperms and 295,383 Angiosperms (belonging to about 485 families and 13,372 genera), considered to be the most recent and vigorous group of plants that have occurred on earth. Angiosperms occupy the majority of the terrestrial space on earth, and are the major components of the world‘s vegetation. Brazil (First) and Colombia (second), both located in the tropics considered to be countries with the most diverse angiosperms floras China (Third) even though the main part of her land is not located in the tropics, the number of angiosperms still occupies the third place in the world. In INDIA there are about 18042 species of flowering plants approximately 320 families, 40 genera and 30,000 species. IUCN Red list Categories: EX –Extinct; EW- Extinct in the Wild-Threatened; CR -Critically Endangered; VU- Vulnerable Angiosperm (Flowering Plants) SPECIES RICHNESS AROUND THE WORLD PLANT CLASSIFICATION Historia Plantarum - the earliest surviving treatise on plants in which Theophrastus listed the names of over 500 plant species. Artificial system of Classification Theophrastus attempted common groupings of folklore combined with growth form such as ( Tree Shrub; Undershrub); or Herb. Or (Annual and Biennials plants) or (Cyme and Raceme inflorescences) or (Archichlamydeae and Meta chlamydeae) or (Upper or Lower ovarian ). -

Pollen Flora of Pakistan -Lxi. Violaceae
Pak. J. Bot., 41(1): 1-5, 2009. POLLEN FLORA OF PAKISTAN -LXI. VIOLACEAE ANJUM PERVEEN AND MUHAMMAD QAISER* Department of Botany, University of Karachi, Karachi, Pakistan *Federal Urdu University of Arts, Science and Technology, Karachi, Pakistan. Abstract Pollen morphology of 5 species of the family Violaceae from Pakistan has been examined by light and scanning electron microscope. Pollen grains are usually radially symmetrical, isopolar, colporate, sub-prolate to prolate-spheroidal. Sexine slightly thicker or thinner than nexine. Tectum mostly densely punctate rarely psilate. On the basis of exine pattern two distinct pollen types viz., Viola pilosa–type and Viola stocksii-type are recognized. Introduction Violaceae is a family with 20 genera and about 800 species (Mabberley, 1987). In Pakistan it is represented by one genus and 17 species (Qaiser & Omer, 1985). Plant perennial herbs, or shrubs leaves simple, alternate rarely opposite, flowers bisexual, zygomorphic or actinomorphic, calyx 5, corolla of 5 petals, anterior petal large and spurred. Androecium of 5 stamens. Gynoecium a compound pistil of 3 united carpels, ovules superior, fruit capsule. The family is of little economic importance except for the garden favorite, Violets, Violas and Pansies. Pollen morphology of the family has been examined by Erdtman (1952), Lobreau- Callen (1977), Moore & Webb (1978) and Dojas et al., (1993). Moore et al., (1991) examined pollen morphology of the genus Viola. Kubitzki (2004) examined the pollen morphology of the family Violaceae. There are no reports on pollen morphology of the family Violaceae from Pakistan. Present investigations are based on the pollen morphology of 5 species representing a single genus of the family Violaceae by light and scanning electron microscope. -

Oral Allergy Syndrome: a Confuence of Immunology and Phylogeny by Merle K
NATIONAL CENTER FOR CASE STUDY TEACHING IN SCIENCE NATIONAL CENTER FOR CASE STUDY TEACHING IN SCIENCE Oral Allergy Syndrome: A Confuence of Immunology and Phylogeny by Merle K. Heidemann, Mike S. Taylor, Amanda Storm, Cassie Dresser-Briggs, Alexa Warwick, and Peter J.T. White Objectives Upon completion of this case study, you should be able to: • Explain the symptoms of allergic reactions in terms of cell biology (immune system cells). • Describe the molecular features of cross reactivity. • Build phylogenetic trees using morphological data. • Build phylogenetic trees using molecular data and interpret two kinds of phylogenetic trees. Part I – Immunology Sam had a history of allergic reactions, including reactions to various plant pollens. Birch pollen elicited a particularly strong reaction, causing him annoying sneezing fts and a sore throat. As he grew to adulthood, he discovered that he was also variably allergic to common foods, including some raw fruits and vegetables, as well as most nuts. Sam was puzzled by his recent food allergies. Also puzzling was the variability in his reactions. He reacted strongly to some foods; others resulted in only a mild itchy throat. He wondered, What exactly causes allergic reactions? Sam was determined to fgure out how and why he reacted to birch pollen and later became allergic to other plant- based food. He also hoped this information might help him avoid other foods that might cause him allergies. First, Sam decided he needed to know something about the cellular bases of allergic reactions. He knew it had some- thing to do with the immune system. He did some basic research on the Internet and here’s what he found: Allergens are bits of protein from an innocuous foreign substance, such as pollen or food. -

Full of Beans: a Study on the Alignment of Two Flowering Plants Classification Systems
Full of beans: a study on the alignment of two flowering plants classification systems Yi-Yun Cheng and Bertram Ludäscher School of Information Sciences, University of Illinois at Urbana-Champaign, USA {yiyunyc2,ludaesch}@illinois.edu Abstract. Advancements in technologies such as DNA analysis have given rise to new ways in organizing organisms in biodiversity classification systems. In this paper, we examine the feasibility of aligning two classification systems for flowering plants using a logic-based, Region Connection Calculus (RCC-5) ap- proach. The older “Cronquist system” (1981) classifies plants using their mor- phological features, while the more recent Angiosperm Phylogeny Group IV (APG IV) (2016) system classifies based on many new methods including ge- nome-level analysis. In our approach, we align pairwise concepts X and Y from two taxonomies using five basic set relations: congruence (X=Y), inclusion (X>Y), inverse inclusion (X<Y), overlap (X><Y), and disjointness (X!Y). With some of the RCC-5 relationships among the Fabaceae family (beans family) and the Sapindaceae family (maple family) uncertain, we anticipate that the merging of the two classification systems will lead to numerous merged solutions, so- called possible worlds. Our research demonstrates how logic-based alignment with ambiguities can lead to multiple merged solutions, which would not have been feasible when aligning taxonomies, classifications, or other knowledge or- ganization systems (KOS) manually. We believe that this work can introduce a novel approach for aligning KOS, where merged possible worlds can serve as a minimum viable product for engaging domain experts in the loop. Keywords: taxonomy alignment, KOS alignment, interoperability 1 Introduction With the advent of large-scale technologies and datasets, it has become increasingly difficult to organize information using a stable unitary classification scheme over time. -
SIGCHI Conference Paper Format
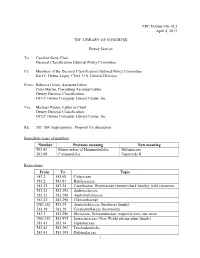
EPC Exhibit 136-19.2 April 4, 2013 THE LIBRARY OF CONGRESS Dewey Section To: Caroline Kent, Chair Decimal Classification Editorial Policy Committee Cc: Members of the Decimal Classification Editorial Policy Committee Karl E. Debus-López, Chief, U.S. General Division From: Rebecca Green, Assistant Editor Giles Martin, Consulting Assistant Editor Dewey Decimal Classification OCLC Online Computer Library Center, Inc. Via: Michael Panzer, Editor in Chief Dewey Decimal Classification OCLC Online Computer Library Center, Inc Re: 583–584 Angiosperms: Proposal for discussion Immediate reuse of numbers Number Previous meaning New meaning 583.43 Minor orders of Hamamelididae Dilleniaceae 583.98 Campanulales Euasterids II Relocations From To Topic 583.2 583.68 Cytinaceae 583.2 583.83 Rafflesiaceae 583.22 583.24 Canellaceae, Winteraceae (winter's bark family), wild cinnamon 583.23 583.292 Amborellaceae 583.23 583.296 Austrobaileyaceae 583.23 583.298 Chloranthaceae [583.26] 583.25 Aristolochiaceae (birthwort family) 583.29 583.28 Ceratophyllaceae (hornworts) 583.3 583.296 Illiciaceae, Schisandraceae, magnolia vine, star anise [583.36] 583.975 Sarraceniaceae (New World pitcher plant family) 583.43 583.34 Eupteleaceae 583.43 583.393 Trochodendrales 583.43 583.395 Didymelaceae 1 583.43 583.44 Cercidiphyllaceae (katsura trees) 583.43 583.46 Casuarinaceae (beefwoods), Myricaceae (wax myrtles), bayberries, candleberry, sweet gale (bog myrtle) 583.43 583.73 Barbeyaceae 583.43 583.786 Leitneria 583.43 583.83 Balanopaceae 583.43 583.942 Eucommiaceae 583.44 -

Biogeography and Diversification of Brassicales
Molecular Phylogenetics and Evolution 99 (2016) 204–224 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Biogeography and diversification of Brassicales: A 103 million year tale ⇑ Warren M. Cardinal-McTeague a,1, Kenneth J. Sytsma b, Jocelyn C. Hall a, a Department of Biological Sciences, University of Alberta, Edmonton, Alberta T6G 2E9, Canada b Department of Botany, University of Wisconsin, Madison, WI 53706, USA article info abstract Article history: Brassicales is a diverse order perhaps most famous because it houses Brassicaceae and, its premier mem- Received 22 July 2015 ber, Arabidopsis thaliana. This widely distributed and species-rich lineage has been overlooked as a Revised 24 February 2016 promising system to investigate patterns of disjunct distributions and diversification rates. We analyzed Accepted 25 February 2016 plastid and mitochondrial sequence data from five gene regions (>8000 bp) across 151 taxa to: (1) Available online 15 March 2016 produce a chronogram for major lineages in Brassicales, including Brassicaceae and Arabidopsis, based on greater taxon sampling across the order and previously overlooked fossil evidence, (2) examine Keywords: biogeographical ancestral range estimations and disjunct distributions in BioGeoBEARS, and (3) determine Arabidopsis thaliana where shifts in species diversification occur using BAMM. The evolution and radiation of the Brassicales BAMM BEAST began 103 Mya and was linked to a series of inter-continental vicariant, long-distance dispersal, and land BioGeoBEARS bridge migration events. North America appears to be a significant area for early stem lineages in the Brassicaceae order. Shifts to Australia then African are evident at nodes near the core Brassicales, which diverged Cleomaceae 68.5 Mya (HPD = 75.6–62.0). -

Calibrated Chronograms, Fossils, Outgroup Relationships, and Root Priors: Re-Examining the Historical Biogeography of Geraniales
bs_bs_banner Biological Journal of the Linnean Society, 2014, 113, 29–49. With 4 figures Calibrated chronograms, fossils, outgroup relationships, and root priors: re-examining the historical biogeography of Geraniales KENNETH J. SYTSMA1,*, DANIEL SPALINK1 and BRENT BERGER2 1Department of Botany, University of Wisconsin, Madison, WI 53706, USA 2Department of Biological Sciences, St. John’s University, Queens, NY 11439, USA Received 26 November 2013; revised 23 February 2014; accepted for publication 24 February 2014 We re-examined the recent study by Palazzesi et al., (2012) published in the Biological Journal of the Linnean Society (107: 67–85), that presented the historical diversification of Geraniales using BEAST analysis of the plastid spacer trnL–F and of the non-coding nuclear ribosomal internal transcribed spacers (ITS). Their study presented a set of new fossils within the order, generated a chronogram for Geraniales and other rosid orders using fossil-based priors on five nodes, demonstrated an Eocene radiation of Geraniales (and other rosid orders), and argued for more recent (Pliocene–Pleistocene) and climate-linked diversification of genera in the five recognized families relative to previous studies. As a result of very young ages for the crown of Geraniales and other rosid orders, unusual relationships of Geraniales to other rosids, and apparent nucleotide substitution saturation of the two gene regions, we conducted a broad series of BEAST analyses that incorporated additional rosid fossil priors, used more accepted rosid ordinal -

JUDD W.S. Et. Al. (1999) Plant Systematics
CHAPTER8 Phylogenetic Relationships of Angiosperms he angiosperms (or flowering plants) are the dominant group of land Tplants. The monophyly of this group is strongly supported, as dis- cussed in the previous chapter, and these plants are possibly sister (among extant seed plants) to the gnetopsids (Chase et al. 1993; Crane 1985; Donoghue and Doyle 1989; Doyle 1996; Doyle et al. 1994). The angio- sperms have a long fossil record, going back to the upper Jurassic and increasing in abundance as one moves through the Cretaceous (Beck 1973; Sun et al. 1998). The group probably originated during the Jurassic, more than 140 million years ago. Cladistic analyses based on morphology, rRNA, rbcL, and atpB sequences do not support the traditional division of angiosperms into monocots (plants with a single cotyledon, radicle aborting early in growth with the root system adventitious, stems with scattered vascular bundles and usually lacking secondary growth, leaves with parallel venation, flow- ers 3-merous, and pollen grains usually monosulcate) and dicots (plants with two cotyledons, radicle not aborting and giving rise to mature root system, stems with vascular bundles in a ring and often showing sec- ondary growth, leaves with a network of veins forming a pinnate to palmate pattern, flowers 4- or 5-merous, and pollen grains predominantly tricolpate or modifications thereof) (Chase et al. 1993; Doyle 1996; Doyle et al. 1994; Donoghue and Doyle 1989). In all published cladistic analyses the “dicots” form a paraphyletic complex, and features such as two cotyle- dons, a persistent radicle, stems with vascular bundles in a ring, secondary growth, and leaves with net venation are plesiomorphic within angio- sperms; that is, these features evolved earlier in the phylogenetic history of tracheophytes. -

Phylogeny of Rosids! ! Rosids! !
Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Alnus - alders A. rubra A. rhombifolia A. incana ssp. tenuifolia Alnus - alders Nitrogen fixation - symbiotic with the nitrogen fixing bacteria Frankia Alnus rubra - red alder Alnus rhombifolia - white alder Alnus incana ssp. tenuifolia - thinleaf alder Corylus cornuta - beaked hazel Carpinus caroliniana - American hornbeam Ostrya virginiana - eastern hophornbeam Phylogeny of Rosids! Rosids! ! ! ! ! Eurosids I Eurosids II Vitaceae Saxifragales Eurosids I:! Eurosids II:! Zygophyllales! Brassicales! Celastrales! Malvales! Malpighiales! Sapindales! Oxalidales! Myrtales! Fabales! Geraniales! Rosales! Cucurbitales! Fagales! After Jansen et al., 2007, Proc. Natl. Acad. Sci. USA 104: 19369-19374! Fagaceae (Beech or Oak family) ! Fagaceae - 9 genera/900 species.! Trees or shrubs, mostly northern hemisphere, temperate region ! Leaves simple, alternate; often lobed, entire or serrate, deciduous -

The Evolutionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 49 2006 The volutE ionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales W. John Kress Smithsonian Institution Chelsea D. Specht Smithsonian Institution; University of California, Berkeley Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Kress, W. John and Specht, Chelsea D. (2006) "The vE olutionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 49. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/49 Zingiberales MONOCOTS Comparative Biology and Evolution Excluding Poales Aliso 22, pp. 621-632 © 2006, Rancho Santa Ana Botanic Garden THE EVOLUTIONARY AND BIOGEOGRAPHIC ORIGIN AND DIVERSIFICATION OF THE TROPICAL MONOCOT ORDER ZINGIBERALES W. JOHN KRESS 1 AND CHELSEA D. SPECHT2 Department of Botany, MRC-166, United States National Herbarium, National Museum of Natural History, Smithsonian Institution, PO Box 37012, Washington, D.C. 20013-7012, USA 1Corresponding author ([email protected]) ABSTRACT Zingiberales are a primarily tropical lineage of monocots. The current pantropical distribution of the order suggests an historical Gondwanan distribution, however the evolutionary history of the group has never been analyzed in a temporal context to test if the order is old enough to attribute its current distribution to vicariance mediated by the break-up of the supercontinent. Based on a phylogeny derived from morphological and molecular characters, we develop a hypothesis for the spatial and temporal evolution of Zingiberales using Dispersal-Vicariance Analysis (DIVA) combined with a local molecular clock technique that enables the simultaneous analysis of multiple gene loci with multiple calibration points. -

The Cannaceae of the World
BLUMEA 53: 247–318 Published on 29 October 2008 http://dx.doi.org/10.3767/000651908X607945 THE CANNACEAE OF THE WORLD H. MAAS-VAN DE KAMER & P.J.M. MAAS Nationaal Herbarium Nederland, Wageningen University branch (Herbarium Vadense), Generaal Foulkesweg 37, 6703 BL Wageningen, The Netherlands SUMMARY This taxonomic treatment includes all Cannaceae of the world. Emphasis is on the 10 species grow- ing in the wild in the Neotropics. Included are chapters on the history of the taxonomy of the family and genera, and on relationships of higher level taxa. Data are provided on vegetative morphology as well as on floral morphology and floral biology. Structure of the inflorescence, the ovary and ovules, fruit and seeds, germination and seedlings are treated in detail. Finally a chapter on uses and vernacular names, a bibliography, and a list of over 200 names ever used in Canna are added. Key words: Cannaceae, anatomy, floral biology, history, uses, taxonomy worldwide, vernacular names. INTRODUCTION In 1970 P.J.M. Maas and his graduate student W. Segeren started studying the genus Canna making a first attempt at a revision of the species in northern South America. The monograph of Kraenzlin of 1912 was outdated and difficult to use because it had been written in Latin. The publication of Segeren & Maas (1971) comprised 10 species of Canna in 2 subgenera (Distemon and Canna). A few years later, when a second graduate student J. van Duuren joined the team, a worldwide monograph of the family Cannaceae was started. Mainly because of the taxonomic and nomenclatural complexity of Canna indica, the work could not be completed earlier. -

ABSTRACTS 117 Systematics Section, BSA / ASPT / IOPB
Systematics Section, BSA / ASPT / IOPB 466 HARDY, CHRISTOPHER R.1,2*, JERROLD I DAVIS1, breeding system. This effectively reproductively isolates the species. ROBERT B. FADEN3, AND DENNIS W. STEVENSON1,2 Previous studies have provided extensive genetic, phylogenetic and 1Bailey Hortorium, Cornell University, Ithaca, NY 14853; 2New York natural selection data which allow for a rare opportunity to now Botanical Garden, Bronx, NY 10458; 3Dept. of Botany, National study and interpret ontogenetic changes as sources of evolutionary Museum of Natural History, Smithsonian Institution, Washington, novelties in floral form. Three populations of M. cardinalis and four DC 20560 populations of M. lewisii (representing both described races) were studied from initiation of floral apex to anthesis using SEM and light Phylogenetics of Cochliostema, Geogenanthus, and microscopy. Allometric analyses were conducted on data derived an undescribed genus (Commelinaceae) using from floral organs. Sympatric populations of the species from morphology and DNA sequence data from 26S, 5S- Yosemite National Park were compared. Calyces of M. lewisii initi- NTS, rbcL, and trnL-F loci ate later than those of M. cardinalis relative to the inner whorls, and sepals are taller and more acute. Relative times of initiation of phylogenetic study was conducted on a group of three small petals, sepals and pistil are similar in both species. Petal shapes dif- genera of neotropical Commelinaceae that exhibit a variety fer between species throughout development. Corolla aperture of unusual floral morphologies and habits. Morphological A shape becomes dorso-ventrally narrow during development of M. characters and DNA sequence data from plastid (rbcL, trnL-F) and lewisii, and laterally narrow in M.