JUDD W.S. Et. Al. (1999) Plant Systematics
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wild Hyacinth (Camassia Scilloides) in Canada
PROPOSED Species at Risk Act Recovery Strategy Series Adopted under Section 44 of SARA Recovery Strategy for the Wild Hyacinth (Camassia scilloides) in Canada Wild Hyacinth 2015 Recommended citation: Environment Canada. 2015. Recovery Strategy for the Wild Hyacinth (Camassia scilloides) in Canada [Proposed]. Species at Risk Act Recovery Strategy Series. Environment Canada, Ottawa. 21 pp. + Annexes. For copies of the recovery strategy, or for additional information on species at risk, including the Committee on the Status of Endangered Wildlife in Canada (COSEWIC) Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the Species at Risk (SAR) Public Registry1. Cover illustration: © Gary Allen Également disponible en français sous le titre « Programme de rétablissement de la camassie faux-scille (Camassia scilloides) au Canada [Proposition] » © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN Catalogue no. Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. 1 http://www.registrelep-sararegistry.gc.ca RECOVERY STRATEGY FOR THE WILD HYACINTH (CAMMASSIA SCILLOIDES) IN CANADA 2015 Under the Accord for the Protection of Species at Risk (1996), the federal, provincial, and territorial governments agreed to work together on legislation, programs, and policies to protect wildlife species at risk throughout Canada. In the spirit of cooperation of the Accord, the Government of Ontario has given permission to the Government of Canada to adopt the Recovery Strategy for the Wild Hyacinth (Camassia scilloides) in Ontario (Part 2) under Section 44 of the Species at Risk Act (SARA). -

Only Write Down the Correct Answer Next to the Appropriate Question Number on Your Answer Sheet
BOT1B10– November 2015 FACULTY OF SCIENCE DEPARTMENT OF BOTANY and PLANT BIOTECHNOLOGY MODULE PLANT DIVERSITY BOT1B10 CAMPUS APK EXAMINATION NOVEMBER 2015 DATE SESSION 14/November/2015 08:30 – 11:30 EXAMINER: PROF A. MOTEETEE INTERNAL MODERATOR: MRS J. WILLIAMSON DURATION: 3 HOURS MARKS: 120 ____________________________________________________________________________________ NUMBER OF PAGES: 11 PAGES INSTRUCTIONS: ANSWER ALL THE QUESTIONS _____________________________________________________________________________ QUESTION 1 [10] Choose an answer that matches the question the best: Only write down the correct answer next to the appropriate question number on your answer sheet. 1.1. Apart from food and beverages, plants provide human beings with: a) Oxygen, nitrogen, construction materials b) Medicines, essential oils, oxygen, fuel c) Paper, wool, cotton, silk d) Herb and spices, carbon, sodium, fodder for animals 1 BOT1B10– November 2015 1.2 Gymnosperms bear their seeds on the surfaces of: a) Leaves b) Cones c) Stems d) Fruits 1.3 Bryophytes have life cycles that depend on what for reproduction? a) Water b) Soil c) Grass d) Sun 1.4 Plants that have xylem and phloem are known as: a) Seed plants b) Photosynthetic plants c) Vascular-plants d) Non-vascular plants 1.5 Which on the following is the stalk by which the leaf blade is attached to the stem? a) Peduncle b) Pedicel c) Inflorescence d) Petiole 1.6 Seed plants use __________ and ___________ to reproduce. a) Pollen and seed b) Seeds and water c) Food and water d) Leaves and petals 1.7 -
1Lecture Notes 2013
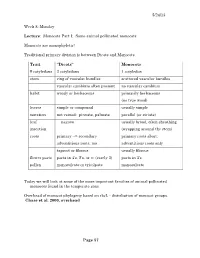
5/24/13 Week 8; Monday Lecture: Monocots Part I: Some animal pollinated monocots Monocots are monophyletic! Traditional primary division is between Dicots and Monocots Trait “Dicots” Monocots # cotyledons 2 cotyledons 1 cotyledon stem ring of vascular bundles scattered vascular bundles vascular cambium often present no vascular cambium habit woody or herbaceous primarily herbaceous (no true wood) leaves simple or compound usually simple venation net veined: pinnate, palmate parallel (or striate) leaf narrow usually broad, often sheathing insertion (wrapping around the stem) roots primary --> secondary primary roots abort; adventitious roots, too adventitious roots only taproot or fibrous usually fibrous flower parts parts in 4’s, 5’s, or ∞ (rarely 3) parts in 3’s pollen monosulcate or tricolpate monosulcate Today we will look at some of the more important families of animal pollinated monocots found in the temperate zone Overhead of monocot phylogeny based on rbcL - distribution of monocot groups. Chase et al. 2000, overhead Page 57 5/24/13 Lab only; limited discussion here. Show: “Plants are Cool, Too” video Araceae - Arum family (109 gen/2830 spp) 1) herbs (some epiphytes) 2) lvs simple or compound; broad and having an apparent petiole (‘pseudo-lamina’) development not same as in a dicot leaf blade 3) calcium oxalate crystals usually present – physical deterrent to herbivory 4) Inflorescence consisting of - spathe - bract (often colorful) surrounding the flowers - spadix - axis on which the flowers are borne (male above; female below, -

SOOS June 2021
Click to join from your computer, tablet SOUTHERN or smartphone: https://www.gotomeet.me/CathyDunn/ ONTARIO 2021-06 or Dial in using your phone: Canada: +1 ORCHID (647) 497-9373 Access Code: 226-011-165 SOCIETY NEWS June 2021, Volume 56, Issue 6 New to GoToMeeting? Get the app now and be Meeting since 1965 ready when your first meeting starts: https://global.gotomeeting.com/install/226011165 June 6Virtual Meeting: Please note that because this meeting is being produced via an internet program the participation capacity is limited to SOOS members only. Be sure to check that you have renewed your membership for Spencer Hauck, Sandhill Botanicals 2020. 2020 Memberships have been extended to the end of 2021. What’s bugging your Orchids? Virtual Show Table Our members are growing and th blooming amazing orchids and you are not shy about sharing Sunday, June 6 , 2021, 1:00 photographs of them. Our virtual show table has doubled in size in less than six months. We often receive over 100 PM (EDT) entries from over 25 different members!! Since we need time to organize the photos and to have them Followed by the judged, photos need to be received by the Wednesday before the Sunday meeting. SOOS Virtual Show Table Submitting photographs to SOOS constitutes permission for Entry Rules: SOOS to publish those images. No remuneration is offered or 1. Take photos between Sun. May 23rd implied. nd and Wed. June 2 . Send your best photos. Points will be given in the usual manner towards the ‘Orchid Grower of the Year’ program for 2. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Some Hyphaene Species from the Botanic Gardens, Catrcutta
I9701 FURTADO: HYPHAENE SomeHyphaene Speciesfrom the Botanic Gardens,Catrcutta C. X. Funrllo Singapore From time to time Hyphaene theboica trations to show its habit, stated that "to is mentioned as a palm that has been H. thebaica was be seen in many a successfullygrown in India. The only gardenof India and Ceylon," (p. 165), Indian speciesthat was commonly mis- an opinion found reiteratedseveral times taken for H. thebaica was distinguished by other writers. by Beccari (1908) under the name of Nevertheless,I was unable to receive H. ind,ica Becc. Nevertheless,Blatter, a fruit from India or Ceylon of a genuine who in his monographicwork The Palms H. thebaica as typified by Martius ol British Ind,ia and Ceylon (1926) ad- (1838). However in the region of mitted 11. inilica as a good speciesand Thebes there seems to occur also a re-describedit with photographic illus- I. Hyphaene Bzssel growing at the Calcutta Ia. Hyphaene Bzssei. Portion of rachiila, part Botanic Garden under the name f1. thebaica. of petiole, hastula and base o{ leaf, {ruit in Photo by T. A. Davis. section. Photo bv Juraimi. PRINCIPES [Vol. 14 2. Hyphaene Bussei at Calcutta. Photo by T. A. Davis. species that is referable to the group "H. namedby Beccari (1924,p.32) as muhiformis" and Beccari's H. thebaica (1924, PL 20) seemsto be referablealso to the latter group, many forms of which are known from Kenya. Apparently, Blatter followed Beccari in identifying "H. H. thebaica with a form of multi- formis," and not with 1/. thebaica (L.) "the Martius; for while he noted that young plants are of slow and precarious growth" in India and Ceylon, older o'much plants were better developed" there than the trees in Egypt (p. -

Traditional Information and Antibacterial Activity of Four Bulbine Species (Wolf)
African Journal of Biotechnology Vol. 10 (2), pp. 220-224, 10 January, 2011 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB10.1435 ISSN 1684–5315 © 2011 Academic Journals Full Length Research Paper Traditional information and antibacterial activity of four Bulbine species (Wolf) R. M. Coopoosamy Department of Nature Conservation, Mangosuthu University of Technology, P O Box 12363, Jacobs4026, Durban, KwaZulu-Natal, South Africa. E-mail: [email protected]. Tel: +27 82 200 3342. Fax: +27 31 907 7665. Accepted 7 December, 2010 Ethnobotanical survey of Bulbine Wolf, (Asphodelaceae) used for various treatment, such as, diarrhea, burns, rashes, blisters and insect bites, was carried out in the Eastern Cape Province of South Africa. Information on the parts used and the methods of preparation was collected through questionnaire which was administered to the herbalists, traditional healers and rural dwellers which indicated the extensive use of Bulbine species. Most uses of Bulbine species closely resemble that of Aloe . Dried leaf bases and leaf sap are the commonest parts of the plants used. Preparations were in the form of decoctions and infusions. Bulbine frutescens was the most frequently and commonly used of the species collected for the treatment of diarrhoea, burns, rashes, blisters, insect bites, cracked lips and mouth ulcers. The leaf, root and rhizome extracts of B. frutescens, Bulbine natalensis, Bulbine latifolia and Bulbine narcissifolia were screened for antibacterial activities to verify their use by traditional healers. Key words: Herbal medicine, diarrhea, medicinal plants, Bulbine species, antibacterial activity. INTRODUCTION Many traditionally used plants are currently being investi- developing countries where traditional medicine plays a gated for various medicinal ailments such as treatment to major role in health care (Farnsworth, 1994; Srivastava et cure stomach aliments, bolding, headaches and many al., 1996). -

Antimicrobial and Chemical Analyses of Selected Bulbine Species
./ /' ANTIMICROBIAL AND CHEMICAL ANALYSES OF SELECTED BULBINE SPECIES BY f' CHUNDERIKA MOCKTAR Submitted in part fulfilment ofthe requirements for the degree of Master of Medical Science (Pharmaceutical Microbiolgy) i,n the Department of Pharmacy in the Faculty of Health Sciences at the Universi1y of Durban-Westville Promotor: Dr S.Y. Essack Co-promotors: Prof. B.C. Rogers Prof. C.M. Dangor .., To my children, Dipika, Jivesh and Samika Page ii sse "" For Shri Vishnu for the guidance and blessings Page iji CONTENTS PAGE Summary IV Acknowledgements VI List ofFigures vu List ofTables X CHAPTER ONE: INTRODUCTION AND LITERATURE REVIEW 1 1.1 Introduction 3 1.1.1 Background and motivation for the study 3 1.1.2 Aims 6 1.2 Literature Review 6 1.2.1 Bacteriology 7 1.2.1.1 Size and shape ofbacteria 7 1.2.1.2 Structure ofBacteria 7 1.2.1.3 The Bacterial Cell Wall 8 1.2.2 Mycology 10 1.2.3 Traditional Medicine in South Africa 12 1.2.3.1 Traditional healers and reasons for consultation 12 1.2.3.2 The integration oftraditional healing systems with western Medicine 13 1.2.3.3 Advantages and Disadvantages ofconsulting traditional healers 14 1.2.4 Useful Medicinal Plants 16 1.2.5 Adverse effects ofplants used medicinally 17 1.2.6 The Bulbine species 19 1.2.6.1 The Asphodelaceae 19 1.2.6.2 Botany ofthe Bulbine species 19 CHAPTER TWO: MATERIALS AND METHODS 27 2.1 Preparation ofthe crude extracts 29 2.1.1 Collection ofthe plant material 30 2.1.2 Organic Extraction 30 2.1.3 Aqueous Extraction 31 2.2 Antibacterial Activities 31 2.2.1 Bacteriology 31 2.2.2 Preparation ofthe Bacterial Cultures 33 2.2.3 Preparation ofthe Agar Plates 33 2.2.4 Preparation ofCrude Extracts 33 2.2.5 Disk Diffusion Method 34 2.2.6 Bore Well Method 34 2.3 Mycology 34 2.3.1 Fungi used in this study 34 2.3.2 Preparation ofFungal Spores 35 2.3.3 Preparation ofC. -

Fruit Trials
Crocosmia AGM by Roundtable FINAL Report 2016 © RHS Author Kirsty Angwin AGM round table coordinator, The Royal Horticultural Society Garden, Wisley, Woking, Surrey, GU23 6QB CROCOSMIA AGM Awards List 2016 AGM by roundtable discussion is a method of awarding AGM when the genus/ plant group in question displays any or all of the following criteria: impractical/ impossible to trial not in the trials plan for the next 5 years proposing plant committee does not contain the expertise to recommend ‘in house’ small number of plants to assess and has the following attributes: current lack of AGMs relevant to today’s gardener outside expertise is identified Present at Meetings: There were no meetings as this round table was conducted and completed by email. The Crocosmia forum was created by the RHS bulb plant committee to assess Crocosmia in 2016. Those on the forum were: Lady Christine Skelmersdale (Chairperson), Mr Bob Brown, Mr Jamie Blake, Mrs Elizabeth MacGregor, Mr Mark Fox, Mr Mark Walsh and Mr John Foley to assess a total of 73 Crocosmia cultivars. It was judged that the forum made up from the RHS Bulb committee, members of the RHS Herbaceous Committee, the National collection holder and nursery specialists had sufficient and comprehensive knowledge to arrive at a sound conclusion on the cultivars awarded. Criteria for voting included: Be available to the public Must be of outstanding excellence for garden decoration or use Good constitution Not require highly specialist growing conditions or care Not be particularly susceptible to any pest or disease Stable in form and colour The Panel recommended the Society's AWARD OF GARDEN MERIT to: Crocosmia x crocosmiiflora ‘Babylon’ Hardiness rating: H4 Description: Large deep orange flowers with a paler centre. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Arthur Monrad Johnson Colletion of Botanical Drawings
http://oac.cdlib.org/findaid/ark:/13030/kt7489r5rb No online items Arthur Monrad Johnson colletion of botanical drawings 1914-1941 Processed by Pat L. Walter. Louise M. Darling Biomedical Library History and Special Collections Division History and Special Collections Division UCLA 12-077 Center for Health Sciences Box 951798 Los Angeles, CA 90095-1798 Phone: 310/825-6940 Fax: 310/825-0465 Email: [email protected] URL: http://www.library.ucla.edu/libraries/biomed/his/ ©2008 The Regents of the University of California. All rights reserved. Arthur Monrad Johnson colletion 48 1 of botanical drawings 1914-1941 Descriptive Summary Title: Arthur Monrad Johnson colletion of botanical drawings, Date (inclusive): 1914-1941 Collection number: 48 Creator: Johnson, Arthur Monrad 1878-1943 Extent: 3 boxes (2.5 linear feet) Repository: University of California, Los Angeles. Library. Louise M. Darling Biomedical Library History and Special Collections Division Los Angeles, California 90095-1490 Abstract: Approximately 1000 botanical drawings, most in pen and black ink on paper, of the structural parts of angiosperms and some gymnosperms, by Arthur Monrad Johnson. Many of the illustrations have been published in the author's scientific publications, such as his "Taxonomy of the Flowering Plants" and articles on the genus Saxifraga. Dr. Johnson was both a respected botanist and an accomplished artist beyond his botanical subjects. Physical location: Collection stored off-site (Southern Regional Library Facility): Advance notice required for access. Language of Material: Collection materials in English Preferred Citation [Identification of item], Arthur Monrad Johnson colletion of botanical drawings (Manuscript collection 48). Louise M. Darling Biomedical Library History and Special Collections Division, University of California, Los Angeles. -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both.