042615A0.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP
Scientific Documentation ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP Not appropriate for regulatory submission. Please visit www.spectrumchemical.com or contact Tech Services for the most up‐to‐date information contained in this information package. Spectrum Chemical Mfg Corp 769 Jersey Avenue New Brunswick, NJ 08901 Phone 732.214.1300 Ver4.05 16.October.2020 ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP Table of Contents Product Specification Certificate of Analysis Sample(s) Safety Data Sheet (SDS) Certification of Current Good Manufacturing Practices (cGMP) Manufacturing Process Flowchart Source Statement BSE/TSE Statement Allergen Statement EU Fragrance Allergen Statement GMO Statement Melamine Statement Nitrosamine Statement Animal Testing Statement Organic Compliance Statement Shelf Life Statement Other Chemicals Statement Elemental Impurities Statement Residual Solvents Statement General Label Information – Sample Label General Lot Numbering System Guidance Kosher Certificate Specification for Dehydrated Alcohol, 200 Proof, Undenatured, USP (ET107) Item Number ET107 Item Dehydrated Alcohol, 200 Proof, Undenatured, USP CAS Number 64-17-5 Molecular Formula C2H5OH Molecular Weight 46.07 MDL Number Synonyms Absolute Ethyl Alcohol ; Anhydrous Ethanol ; Ethanol ; Grain Derived Alcohol Test Specification Min Max ASSAY (by VOLUME) 99.5 % NOT MORE OPALESENT CLARITY OF SOLUTION THAN STANDARD NOT MORE INTENSE THAN COLOR OF SOLUTION STANDARD ACIDITY OR ALKALINITY SOLUTION IS PINK SPECIFIC GRAVITY @ 15.56oC 0.7962 UV ABSORPTION: 240 nm 0.40 250 - 260 nm 0.30 270 - 340 nm 0.10 ORGANIC IMPURITIES: METHANOL 200 μL/L ACETALDEHYDE AND ACETAL 10 μL/L BENZENE 2 μL/L SUM OF ALL OTHER IMPURITIES 300 μL/L LIMIT OF NONVOLATILE RESIDUE 2.5 mg ELEMENTAL IMPURITIES AS REPORTED IDENTIFICATION A 0.7962 SPECTRUM MATCHES IDENTIFICATION B REFERENCE IDENTIFICATION (C) LIMIT OF METHANOL 200 μL/L CERTIFIED KOSHER APPEARANCE EXPIRATION DATE DATE OF MANUFACTURE RESIDUAL SOLVENTS AS REPORTED CLASS 3 (solvent) / 1-PROPANOL . -

Lesson 3 Practice for Naming and Predicting Formulae of Compounds
Title: Lesson 3 Practice for Naming and Predicting formulae of Compounds Objectives: Become proficient at naming and predicting formulae of chemical compounds. Specific Learner Outcomes: SWAT: Explain, using the periodic table, how and why elements combine to form compounds in specific ratios Predict formulas and write names for ionic and molecular compounds and common acids (sulfuric, hydrochloric etc) Activities: Worksheets External Resources: Visions 244-255, 284-292 AWC 89-104 Nelson 73-88 «First_» «Last» Predicting Formulae for Compounds Remember the first step is to determine whether a compound is ionic or molecular! Ionic compounds have a metal and a nonmetal while molecular have two nonmetals. 1. calcium phosphide 21. potassium carbonate 2. cesium oxide 22. lead(IV) chloride 3. manganese(iv) oxide 23. tin(II) bromide 4. iron(II) sulfide 24. ammonium hydroxide 5. potassium permanganate 25. iron(II) hydroxide 6. silver chloride 26. carbon dioxide 7. copper(II) hydroxide 27. dinitrogen pentoxide 8. ammonium sulfide 28. silver oxide 9. nickel(II) bromide 29. aluminum nitride 10. iron(II) oxide 30. manganese(II) hydroxide 11. mercury(I) sulfate 31. ammonium carbonate 12. iron(III) oxide 32. aluminum oxide 13. magnesium phosphide 33. antimony(v) sulfide 14. zinc hydride 34. calcium phosphate 15. diphosphorous pentoxide 35. cesium carbonate 16. aluminum phosphate 36. silver chromate 17. copper(II) nitrate 37. magnesium sulphate 18. nitrogen dioxide 38. chromium(III) phosphide 19. phosphorus trichloride 39. cobalt(III) nitrate 20. sodium phosphide 40. zinc iodide 41. iron(II) fluoride 66. copper(II) iodide 42. nickel(II) selenide 67. silver nitride 43. lithium oxide 68. -

Theoretical Calculations on the Structural, Electronic and Optical
Theoretical calculations on the structural, electronic and optical properties of bulk silver nitrides Mohammed S. H. Suleiman1,2, ∗ and Daniel P. Joubert1, † 1School of Physics, University of the Witwatersrand, Johannesburg, South Africa. 2Department of Physics, Sudan University of Science and Technology, Khartoum, Sudan. (Dated: January 1, 2013) We present a first-principles investigation of structural, electronic and optical properties of bulk crystalline Ag3N, AgN and AgN2 based on density functional theory (DFT) and many-body pertur- bation theory. The equation of state (EOS), energy-optimized geometries, cohesive and formation energies, and bulk modulus and its pressure derivative of these three stoichiometries in a set of twenty different structures have been studied. Band diagrams and total and orbital-resolved density of states (DOS) of the most stable phases have been carefully examined. Within the random-phase approximation (RPA) to the dielectric tensor, the single-particle spectra of the quasi electrons and quasi holes were obtained via the GW approximation to the self-energy operator, and optical spec- tra were calculated. The results obtained were compared with experiment and with previously performed calculations. CONTENTS published works5. Due to its early discovery, copper ni- tride may now be considered as the most investigated 6 I. Introduction 1 among the late TMNs . On the other hand, the nitride of silver, the next el- II. Calculation Methods 2 ement to copper in group 11 of the periodic table, has 7,8 A. Stoichiometries and Crystal Structures 2 been known for more than two centuries . However, de- B. Electronic Relaxation Details 2 spite its earlier discovery, silver nitride may be the least theoretically studied solid in the late TMNs family. -

Study on the Formation and the Decomposition of Agn3 and A
Study on the formation and the decomposition of AgN3 and a hypothetical compound ReN3 by using density functional calculations. G. Soto. Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología Km 107 carretera Tijuana-Ensenada, Ensenada Baja California, México. Corresponding Author: G. Soto. CNyN-UNAM P.O. Box 439036, San Ysidro, CA 92143-9036, USA Tel: +52+646+1744602, Fax: +52+646+1744603 E-Mail: [email protected] Abstract We present a comparative study between ReN3 and AgN3 by using density functional theory. The ReN3 is a hypothetical compound proposed by us to interpret the Re to Re interplanar spacing of thin films grown by sputtering. Both, the AgN3 as the ReN3, are calculated as positive enthalpy compounds. The enthalpy might give a clue about the spontaneous decomposition of the solid form, but it cannot be interpreted as a restriction of its synthesizability. As from the calculated total-energy, we discuss the route for the formation of AgN3 starting from atomic species in aqueous solution. We propose that their synthesizability is conditioned by the energy of free nitrogen atoms, and the kinetics of reaction. We conclude that the intrinsic stability of a certain atomic arrangement depends only of the equilibrium of atomic forces, and not from the energy value associated with that structure. 1 1. Introduction Predicting new solids based solely on computer calculations is one of the main challenges of materials science. Achieving this would mean a giant step forward as it would save many hours of fruitless efforts. Although there has been significant progress[1], it is still early to sing praises. -

Nitrous Acid)
5. Nitrogen Group Content 5.1 Occurrence 5.2 Group Properties Group 5.3 Physical Properties 15 or VA 5.4 Syntheses 7 1772 5.5 Chemical Behaviour N 15 5.6 Applications 1669 5.7 Chemistry of Elemental Nitrogen P 33 5.8 Compounds Made of Nitrogen and Hydrogen Antique 5.9 Nitrogen Compounds with Oxygen As 51 5.10 Nitrogen Compounds with Halides Antique Sb 5.11 Phosphorus/Hydrogen Compounds 83 1753 5.12 Phosphorus Oxides Bi 5.13 Oxo Acids of Phosphorus 115 2003 5.14 Phosphorus Compounds with Halides Mc 5.15 Arsenic, Antimony and Bismuth 5.16 Biological Aspects „Penteles“ Inorganic Chemistry I Slide 1 Prof. Dr. T. Jüstel 5.1 Occurrence Außer Phosphor kommen alle Pentele auch elementar (gediegen) vor Nitrogen (nitrogenium) N2 (78.1% in the air) NaNO3 Chile saltpetre KNO3 Saltpetre Phosphorus (phosphoros) Ca5(PO4)3(OH,F) Apatite greek: lightbearer Ca3(PO4)2 Phosphorite . Fe3(PO4)2 8H2O Vivianite Arsenic (arsenikos) FeAsS Arsenopyrite greek: mineral name As4S4 Realgar As4S3 Antimony (antimonium) Sb native Stibium = greek mineral name Sb2S3 Bismuth (bismutum) Bi native german: Wismut = Mutung “in the meadows” Bi2S3 Inorganic Chemistry I Slide 2 Prof. Dr. T. Jüstel 5.2 Group Properties Whereas Nitrogen Exhibits the Typical Properties of A Non-Metal, Bismuth Is Solely Metallic N P As Sb Bi Atomic number 7 15 33 51 83 Electronic [He] [Ne] [Ar] [Kr] [Xe]4f14 configuration 2s22p3 3s23p3 3d104s24p3 4d105s25p3 5d106s26p3 Electronegativity 3.0 2.1 2.2 1.8 1.7 Ionisation energy [eV] 14.5 11.0 9.8 8.6 7.3 Electronic affinity [eV] -0.3 0.6 0.7 0.6 > 0.7 Character of oxides acidic acidic amphoteric amphoteric alkaline Oxidation states -3, ...…, +5 With increasing atomic number, the oxidation state +3 becomes more stable, whilst the oxidation state +5 becomes instable. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

United States Patent Office Patented Aug
3,832,364 United States Patent Office Patented Aug. 27, 1974. 2 zene, o-, m- and p-dichlorobenzene, anisole, methyl. 3,832,364 benzyl ether, n-propyl phenyl ether, m-ethyl-anisole, 4 AMINATION OF AROMATIC COMPOUNDS IN methyldiphenyl ether, di-p-tolyl ether, ethyl 2-cyclohexyl LIQUID HYDROGEN FLUORIDE Dale Robert Coulson, Wilmington, Del assignor to E. I. phenyl ether, n-heptyl phenyl ether, isobutyl phenyl ether, du Pont de Nemours and Company, Wilmington, Del. 5 cyclohexyl phenyl ether, n-octyl phenyl ether, n-octyl p No Drawing. Filed May 25, 1972, Ser. No. 256,770 tolyl ether, n-octyl m-tolyl ether, decyl phenyl ether, 1 Int. C. C07c87/50, 97/12 benzylethylheptyl ether, benzoic acid, p-toluic acid, p U.S. C. 260-378 26 Claims chlorobenzoic acid, phthalic acid, nitrobenzene, m-dinitro benzene, o-, m- and p-nitrotoluene, acetophenone, methyl O benzyl ketone, p-methylacetophenone, 3-phenyl-2-buta ABSTRACT OF THE DISCLOSURE none, propriophenone, 2-methyl-1-tetralone, phenyl. n Disclosed herein is a process for making aromatic butyl ketone, benzophenone, p-methylbenzophenone, di amines from benzene or substituted benzene and an o-tolyl ketone, phenyl isobutyl ketone, phenyl n-hexyl aminating agent via direct amination in liquid hydrogen ketone, phenyl n - undecyl ketone, anthraquinone, 2 - fluoride medium as solvent and catalyst. 15 methylanthraquinone, and 1-methyl-4-isopropylanthra quinone. Preferred aromatic compounds are those containing BACKGROUND OF THE INVENTION two or fewer substituents. Especially preferred com 1. Field of the Invention pounds are those containing two or fewer substituents 20 selected from halogen and alkyl of up to four carbons. -

Material Safety Data Sheet Ethyl Alcohol 200 Proof MSDS
Material Safety Data Sheet Ethyl alcohol 200 Proof MSDS Section 1: Chemical Product and Company Identification Product Name: Ethyl alcohol 200 Proof Contact Information: Catalog Codes: SLE2248, SLE1357 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: 64-17-5 Houston, Texas 77396 US Sales: 1-800-901-7247 RTECS: KQ6300000 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Ethyl alcohol 200 Proof Order Online: ScienceLab.com CI#: Not applicable. CHEMTREC (24HR Emergency Telephone), call: 1-800-424-9300 Synonym: Ethanol; Absolute Ethanol; Alcohol; Ethanol 200 proof; Ethyl Alcohol, Anhydrous; Ethanol, undenatured; International CHEMTREC, call: 1-703-527-3887 Dehydrated Alcohol; Alcohol For non-emergency assistance, call: 1-281-441-4400 Chemical Name: Ethyl Alcohol Chemical Formula: CH3CH2OH Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Ethyl alcohol 200 Proof 64-17-5 100 Toxicological Data on Ingredients: Ethyl alcohol 200 Proof: ORAL (LD50): Acute: 7060 mg/kg [Rat]. 3450 mg/kg [Mouse]. VAPOR (LC50): Acute: 20000 ppm 8 hours [Rat]. 39000 mg/m 4 hours [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation. Slightly hazardous in case of skin contact (permeator), of ingestion. Potential Chronic Health Effects: Slightly hazardous in case of skin contact (sensitizer). CARCINOGENIC EFFECTS: A4 (Not classifiable for human or animal.) by ACGIH. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. TERATOGENIC EFFECTS: Classified PROVEN for human. DEVELOPMENTAL TOXICITY: Classified Development toxin [PROVEN]. Classified Reproductive system/toxin/female, Reproductive system/toxin/male [POSSIBLE]. -

Harrison School District Chemical Hygiene Plan
Harrison School District Chemical Hygiene Plan Updated & Approved by Mark Wilsey on 10-11-2019 Table of Contents Introduction and Overview .................................................................................................... 3 Section I: Annual Review ................................................................................................................ 3 Section II: Laboratory Hazardous Materials and Chemical Management .......................................... 3 Section II (a): Administrative Positions and Duties ......................................................................................... 3 Section III: Purchasing Procedures .................................................................................................. 4 Section IV: On-Site Hazardous Materials and Chemical Management ............................................... 5 Management of Chemicals .................................................................................................... 8 Acquisition of Chemicals ................................................................................................................. 8 General Rules and Procedures ........................................................................................................ 8 Inventory and Tracking ................................................................................................................... 9 Safety Data Sheets ........................................................................................................................ -

Attached Are the MSDS Lists for Various Chemicals Used During the Wet Plate Collodion / Platinum Palladium Courses at Oxbow
Attached are the MSDS lists for various chemicals used during the Wet Plate Collodion / Platinum Palladium courses at OxBow. Many of the materials for this course are highly toxic and therefore thorough communication and understanding about this aspect of wet plate collodion. Safety is a necessity. It is our first priority and concern to having a productive community throughout our courses. Please contact us should you have any questions or concerns, we will be happy to answer. Thank you, Jaclyn Silverman and Robert Clarke-Davis MSDS Lists: Wet-plate collodion * you will be required to use nitrile gloves, there is no exception to this rule. SAFETY DATA SHEET Preparation Date: No data available Revision Date: 6/17/2015 Revision Number: G1 Product identifier Product code: CO120 Product Name: COLLODION, USP Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2 Reproductive toxicity Category 1B Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 1 Flammable liquids Category 1 Label elements Product code: CO120 Product name: COLLODION, USP 1 / 16 Danger Hazard statements Harmful if swallowed Causes skin irritation Causes serious eye irritation May damage fertility or the unborn child May cause respiratory irritation. -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M. -
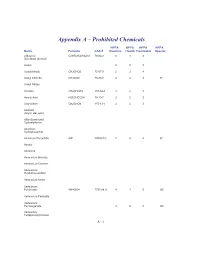
Appendix a – Prohibited Chemicals
Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special 2-Butanol C2H5CH(OH)CH3 78-92-2 0 1 3 (Sec-Butyl Alcohol) Acetal 023 Acetaldehyde CH3CHO5 75-07-0 2 3 4 Acetyl Chloride CH3COCl 75-36-5 2 3 3 W Acetyl Nitrate Acrolein CH2CHCHO 107-02-8 3 4 3 Acrylic Acid H2CCHCO2H 79-10-7 2 2 2 Acrylonitrile CH2CHCN 107-13-1 2 4 3 Alcohols (Allylic, Benzylic) Alkly-Substituted Cycloaliphatics Aluminum Hydrophosphide Aluminum Phosphide AlP 20859-73- 2 4 4 W Amatol Ammonal Ammonium Bromate Ammonium Chlorate Ammonium Hexanitrocobaltate Ammonium Nitrite Ammonium Perchlorate NH4ClO4 7790-98-9 4 1 0 OX Ammonium Periodate Ammonium Permanganate 300 OX Ammonium Tetraperoxychromate A - 1 Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special Antimony Compounds Arsenic And Arsenic Compounds Azides Azidocarbonyl Guanidine Barium Ba 2 2 1 W Barium Chlorate Ba(ClO3)2*H2O 13477-00- 1 2 0 OX Barium Oxide (Anhydrous) BaO 1304-28-5 2 3 0 Barium Peroxide BaO2 1304-29-6 0 1 0 OX Benzene C6H6 71-43-2 0 2 3 Benzene Diazonium Chloride Benzotriazole C6H5N3 95-14-7 0 2 1 Benzoyl Peroxide (C6H5CO)2O2 94-36-0 4 1 4 OX Benzyl Alcohol C6H5CH2OH 100-51-6 0 2 1 Bismuth Nitrate Bi(NO3)3*5H2O 10035-06- 3 1 0 OX Borane,Boranes, Diboranes Boron Tribromide 230 W Boron Trifluoride 140 Bromine Pentafluoride Brf5 7789-30-2 3 4 0 W,O Bromine Trifluoride 3 4 0 W,O Butadiene C4H6/CH2=(CH)2=CH 106-99-0 0 2 4 Butenetroil Trinitrate Cadmium and Cadmium Compounds Calcium Nitrate, Anhydrous Ca(NO3)2