Theoretical Calculations on the Structural, Electronic and Optical
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP
Scientific Documentation ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP Not appropriate for regulatory submission. Please visit www.spectrumchemical.com or contact Tech Services for the most up‐to‐date information contained in this information package. Spectrum Chemical Mfg Corp 769 Jersey Avenue New Brunswick, NJ 08901 Phone 732.214.1300 Ver4.05 16.October.2020 ET107, Dehydrated Alcohol, 200 Proof, Undenatured, USP Table of Contents Product Specification Certificate of Analysis Sample(s) Safety Data Sheet (SDS) Certification of Current Good Manufacturing Practices (cGMP) Manufacturing Process Flowchart Source Statement BSE/TSE Statement Allergen Statement EU Fragrance Allergen Statement GMO Statement Melamine Statement Nitrosamine Statement Animal Testing Statement Organic Compliance Statement Shelf Life Statement Other Chemicals Statement Elemental Impurities Statement Residual Solvents Statement General Label Information – Sample Label General Lot Numbering System Guidance Kosher Certificate Specification for Dehydrated Alcohol, 200 Proof, Undenatured, USP (ET107) Item Number ET107 Item Dehydrated Alcohol, 200 Proof, Undenatured, USP CAS Number 64-17-5 Molecular Formula C2H5OH Molecular Weight 46.07 MDL Number Synonyms Absolute Ethyl Alcohol ; Anhydrous Ethanol ; Ethanol ; Grain Derived Alcohol Test Specification Min Max ASSAY (by VOLUME) 99.5 % NOT MORE OPALESENT CLARITY OF SOLUTION THAN STANDARD NOT MORE INTENSE THAN COLOR OF SOLUTION STANDARD ACIDITY OR ALKALINITY SOLUTION IS PINK SPECIFIC GRAVITY @ 15.56oC 0.7962 UV ABSORPTION: 240 nm 0.40 250 - 260 nm 0.30 270 - 340 nm 0.10 ORGANIC IMPURITIES: METHANOL 200 μL/L ACETALDEHYDE AND ACETAL 10 μL/L BENZENE 2 μL/L SUM OF ALL OTHER IMPURITIES 300 μL/L LIMIT OF NONVOLATILE RESIDUE 2.5 mg ELEMENTAL IMPURITIES AS REPORTED IDENTIFICATION A 0.7962 SPECTRUM MATCHES IDENTIFICATION B REFERENCE IDENTIFICATION (C) LIMIT OF METHANOL 200 μL/L CERTIFIED KOSHER APPEARANCE EXPIRATION DATE DATE OF MANUFACTURE RESIDUAL SOLVENTS AS REPORTED CLASS 3 (solvent) / 1-PROPANOL . -

Lesson 3 Practice for Naming and Predicting Formulae of Compounds
Title: Lesson 3 Practice for Naming and Predicting formulae of Compounds Objectives: Become proficient at naming and predicting formulae of chemical compounds. Specific Learner Outcomes: SWAT: Explain, using the periodic table, how and why elements combine to form compounds in specific ratios Predict formulas and write names for ionic and molecular compounds and common acids (sulfuric, hydrochloric etc) Activities: Worksheets External Resources: Visions 244-255, 284-292 AWC 89-104 Nelson 73-88 «First_» «Last» Predicting Formulae for Compounds Remember the first step is to determine whether a compound is ionic or molecular! Ionic compounds have a metal and a nonmetal while molecular have two nonmetals. 1. calcium phosphide 21. potassium carbonate 2. cesium oxide 22. lead(IV) chloride 3. manganese(iv) oxide 23. tin(II) bromide 4. iron(II) sulfide 24. ammonium hydroxide 5. potassium permanganate 25. iron(II) hydroxide 6. silver chloride 26. carbon dioxide 7. copper(II) hydroxide 27. dinitrogen pentoxide 8. ammonium sulfide 28. silver oxide 9. nickel(II) bromide 29. aluminum nitride 10. iron(II) oxide 30. manganese(II) hydroxide 11. mercury(I) sulfate 31. ammonium carbonate 12. iron(III) oxide 32. aluminum oxide 13. magnesium phosphide 33. antimony(v) sulfide 14. zinc hydride 34. calcium phosphate 15. diphosphorous pentoxide 35. cesium carbonate 16. aluminum phosphate 36. silver chromate 17. copper(II) nitrate 37. magnesium sulphate 18. nitrogen dioxide 38. chromium(III) phosphide 19. phosphorus trichloride 39. cobalt(III) nitrate 20. sodium phosphide 40. zinc iodide 41. iron(II) fluoride 66. copper(II) iodide 42. nickel(II) selenide 67. silver nitride 43. lithium oxide 68. -

Study on the Formation and the Decomposition of Agn3 and A
Study on the formation and the decomposition of AgN3 and a hypothetical compound ReN3 by using density functional calculations. G. Soto. Universidad Nacional Autónoma de México, Centro de Nanociencias y Nanotecnología Km 107 carretera Tijuana-Ensenada, Ensenada Baja California, México. Corresponding Author: G. Soto. CNyN-UNAM P.O. Box 439036, San Ysidro, CA 92143-9036, USA Tel: +52+646+1744602, Fax: +52+646+1744603 E-Mail: [email protected] Abstract We present a comparative study between ReN3 and AgN3 by using density functional theory. The ReN3 is a hypothetical compound proposed by us to interpret the Re to Re interplanar spacing of thin films grown by sputtering. Both, the AgN3 as the ReN3, are calculated as positive enthalpy compounds. The enthalpy might give a clue about the spontaneous decomposition of the solid form, but it cannot be interpreted as a restriction of its synthesizability. As from the calculated total-energy, we discuss the route for the formation of AgN3 starting from atomic species in aqueous solution. We propose that their synthesizability is conditioned by the energy of free nitrogen atoms, and the kinetics of reaction. We conclude that the intrinsic stability of a certain atomic arrangement depends only of the equilibrium of atomic forces, and not from the energy value associated with that structure. 1 1. Introduction Predicting new solids based solely on computer calculations is one of the main challenges of materials science. Achieving this would mean a giant step forward as it would save many hours of fruitless efforts. Although there has been significant progress[1], it is still early to sing praises. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Material Safety Data Sheet Ethyl Alcohol 200 Proof MSDS
Material Safety Data Sheet Ethyl alcohol 200 Proof MSDS Section 1: Chemical Product and Company Identification Product Name: Ethyl alcohol 200 Proof Contact Information: Catalog Codes: SLE2248, SLE1357 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: 64-17-5 Houston, Texas 77396 US Sales: 1-800-901-7247 RTECS: KQ6300000 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Ethyl alcohol 200 Proof Order Online: ScienceLab.com CI#: Not applicable. CHEMTREC (24HR Emergency Telephone), call: 1-800-424-9300 Synonym: Ethanol; Absolute Ethanol; Alcohol; Ethanol 200 proof; Ethyl Alcohol, Anhydrous; Ethanol, undenatured; International CHEMTREC, call: 1-703-527-3887 Dehydrated Alcohol; Alcohol For non-emergency assistance, call: 1-281-441-4400 Chemical Name: Ethyl Alcohol Chemical Formula: CH3CH2OH Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Ethyl alcohol 200 Proof 64-17-5 100 Toxicological Data on Ingredients: Ethyl alcohol 200 Proof: ORAL (LD50): Acute: 7060 mg/kg [Rat]. 3450 mg/kg [Mouse]. VAPOR (LC50): Acute: 20000 ppm 8 hours [Rat]. 39000 mg/m 4 hours [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation. Slightly hazardous in case of skin contact (permeator), of ingestion. Potential Chronic Health Effects: Slightly hazardous in case of skin contact (sensitizer). CARCINOGENIC EFFECTS: A4 (Not classifiable for human or animal.) by ACGIH. MUTAGENIC EFFECTS: Mutagenic for mammalian somatic cells. Mutagenic for bacteria and/or yeast. TERATOGENIC EFFECTS: Classified PROVEN for human. DEVELOPMENTAL TOXICITY: Classified Development toxin [PROVEN]. Classified Reproductive system/toxin/female, Reproductive system/toxin/male [POSSIBLE]. -

Harrison School District Chemical Hygiene Plan
Harrison School District Chemical Hygiene Plan Updated & Approved by Mark Wilsey on 10-11-2019 Table of Contents Introduction and Overview .................................................................................................... 3 Section I: Annual Review ................................................................................................................ 3 Section II: Laboratory Hazardous Materials and Chemical Management .......................................... 3 Section II (a): Administrative Positions and Duties ......................................................................................... 3 Section III: Purchasing Procedures .................................................................................................. 4 Section IV: On-Site Hazardous Materials and Chemical Management ............................................... 5 Management of Chemicals .................................................................................................... 8 Acquisition of Chemicals ................................................................................................................. 8 General Rules and Procedures ........................................................................................................ 8 Inventory and Tracking ................................................................................................................... 9 Safety Data Sheets ........................................................................................................................ -

Attached Are the MSDS Lists for Various Chemicals Used During the Wet Plate Collodion / Platinum Palladium Courses at Oxbow
Attached are the MSDS lists for various chemicals used during the Wet Plate Collodion / Platinum Palladium courses at OxBow. Many of the materials for this course are highly toxic and therefore thorough communication and understanding about this aspect of wet plate collodion. Safety is a necessity. It is our first priority and concern to having a productive community throughout our courses. Please contact us should you have any questions or concerns, we will be happy to answer. Thank you, Jaclyn Silverman and Robert Clarke-Davis MSDS Lists: Wet-plate collodion * you will be required to use nitrile gloves, there is no exception to this rule. SAFETY DATA SHEET Preparation Date: No data available Revision Date: 6/17/2015 Revision Number: G1 Product identifier Product code: CO120 Product Name: COLLODION, USP Other means of identification Synonyms: No information available CAS #: Mixture RTECS # Not available CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: No information available. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Martin LaBenz (West Coast) Contact Person: Ibad Tirmiz (East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Acute toxicity - Oral Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2 Reproductive toxicity Category 1B Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 1 Flammable liquids Category 1 Label elements Product code: CO120 Product name: COLLODION, USP 1 / 16 Danger Hazard statements Harmful if swallowed Causes skin irritation Causes serious eye irritation May damage fertility or the unborn child May cause respiratory irritation. -
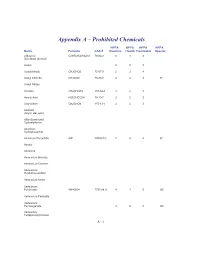
Appendix a – Prohibited Chemicals
Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special 2-Butanol C2H5CH(OH)CH3 78-92-2 0 1 3 (Sec-Butyl Alcohol) Acetal 023 Acetaldehyde CH3CHO5 75-07-0 2 3 4 Acetyl Chloride CH3COCl 75-36-5 2 3 3 W Acetyl Nitrate Acrolein CH2CHCHO 107-02-8 3 4 3 Acrylic Acid H2CCHCO2H 79-10-7 2 2 2 Acrylonitrile CH2CHCN 107-13-1 2 4 3 Alcohols (Allylic, Benzylic) Alkly-Substituted Cycloaliphatics Aluminum Hydrophosphide Aluminum Phosphide AlP 20859-73- 2 4 4 W Amatol Ammonal Ammonium Bromate Ammonium Chlorate Ammonium Hexanitrocobaltate Ammonium Nitrite Ammonium Perchlorate NH4ClO4 7790-98-9 4 1 0 OX Ammonium Periodate Ammonium Permanganate 300 OX Ammonium Tetraperoxychromate A - 1 Appendix A – Prohibited Chemicals NFPA NFPA NFPA NFPA Name Formula CAS #Reactive Health Flammable Special Antimony Compounds Arsenic And Arsenic Compounds Azides Azidocarbonyl Guanidine Barium Ba 2 2 1 W Barium Chlorate Ba(ClO3)2*H2O 13477-00- 1 2 0 OX Barium Oxide (Anhydrous) BaO 1304-28-5 2 3 0 Barium Peroxide BaO2 1304-29-6 0 1 0 OX Benzene C6H6 71-43-2 0 2 3 Benzene Diazonium Chloride Benzotriazole C6H5N3 95-14-7 0 2 1 Benzoyl Peroxide (C6H5CO)2O2 94-36-0 4 1 4 OX Benzyl Alcohol C6H5CH2OH 100-51-6 0 2 1 Bismuth Nitrate Bi(NO3)3*5H2O 10035-06- 3 1 0 OX Borane,Boranes, Diboranes Boron Tribromide 230 W Boron Trifluoride 140 Bromine Pentafluoride Brf5 7789-30-2 3 4 0 W,O Bromine Trifluoride 3 4 0 W,O Butadiene C4H6/CH2=(CH)2=CH 106-99-0 0 2 4 Butenetroil Trinitrate Cadmium and Cadmium Compounds Calcium Nitrate, Anhydrous Ca(NO3)2 -

2 1 0 Material Safety Data Sheet
He a lt h 1 2 Fire 2 1 0 Re a c t iv it y 0 Pe rs o n a l Pro t e c t io n H Material Safety Data Sheet Methylene Blue Solution, Loeffler MSDS Section 1: Chemical Product and Company Identification Product Name: Methylene Blue Solution, Loeffler Contact Information: Catalog Codes: SLM3328 Sciencelab.com, Inc. 14025 Smith Rd. CAS#: Mixture. Houston, Texas 77396 RTECS: Not applicable. US Sales: 1-800-901-7247 International Sales: 1-281-441-4400 TSCA: TSCA 8(b) inventory: Potassium hydroxide; Ethyl alcohol 200 Proof; Water Order Online: ScienceLab.com CI#: Not applicable. CHEMTREC (24HR Emergency Telephone), call: 1-800-424-9300 Synonym: Methylene Blue-Loeffler's Alkaline International CHEMTREC, call: 1-703-527-3887 Chemical Name: Not applicable. For non-emergency assistance, call: 1-281-441-4400 Chemical Formula: Not applicable. Section 2: Composition and Information on Ingredients Composition: Name CAS # % by Weight Potassium hydroxide 1310-58-3 <1 Ethyl alcohol 200 Proof 64-17-5 25-30 Water 7732-18-5 70-75 Methylene Blue CI52015 7220-79-3 <1 Toxicological Data on Ingredients: Ethyl alcohol 200 Proof: ORAL (LD50): Acute: 7060 mg/kg [Rat.]. 3450 mg/kg [Mouse]. VAPOR (LC50): Acute: 20000 ppm 8 hours [Rat]. 39000 mg/m 4 hours [Mouse]. Section 3: Hazards Identification Potential Acute Health Effects: Hazardous in case of skin contact (irritant, permeator), of eye contact (irritant), of ingestion. Potential Chronic Health Effects: CARCINOGENIC EFFECTS: Classified PROVEN by State of California Proposition 65 [Ethyl alcohol 200 Proof]. Classified A4 (Not classifiable for human or animal.) by ACGIH [Ethyl alcohol 200 Proof]. -

THE MONATOMIC IONS! 1. What Is the Formula for Silver? Ag 2. What Is
Name: ______________________________ THE MONATOMIC IONS! 1. What is the formula for silver? Ag+ 22. What is the formula for cobalt (II)? Co2+ 2. What is the formula for cadmium? Cd2+ 23. What is the formula for chromium (II)? Cr2+ 3. What is the formula for manganese (II)? Mn2+ 24. What is the formula for copper (II)? Cu2+ 4. What is the formula for nickel (II)? Ni2+ 25. What is the formula for tin (IV)? Sn4+ 5. What is the formula for chromous? Cr2+ 26. What is the formula for lead (IV)? Pb4+ 6. What is the formula for zinc? Zn2+ 27. What is the formula for iron (III)? Fe3+ 2+ 2+ 7. What is the formula for cobaltous? Co 28. What is the formula for mercury (I)? Hg2 8. What is the formula for cuprous? Cu+ 29. What is the formula for lead (II)? Pb2+ 9. What is the formula for ferrous? Fe2+ 30. What is the formula for mercury (II)? Hg2+ 2+ 2+ 10. What is the formula for mercurous? Hg2 31. What is the formula for iron (II)? Fe 11. What is the formula for stannous? Sn2+ 32. What is the formula for copper (I)? Cu+ 12. What is the formula for plumbous? Pb2+ 33. What is the formula for tin (II)? Sn2+ 13. What is the formula for chromic? Cr3+ 34. What is the formula for fluoride? F- 14. What is the formula for cobaltic? Co3+ 35. What is the formula for chloride? Cl- 15. What is the formula for cupric? Cu2+ 36. What is the formula for hydride? H- 16. -

SILVER COMPOUNDS 1. Introduction Silver, a White, Lustrous Metal
SILVER COMPOUNDS 1. Introduction Silver, a white, lustrous metal, slightly less malleable and ductile than gold (see GOLD AND GOLD COMPOUNDS), has high thermal and electrical conductivity (see SIL- VER AND SILVER ALLOYS). Most silver compounds are made from silver nitrate [7761- 88-8], AgNO3, which is prepared from silver metal. Some silver metal is found in Nature, frequently alloyed with other metals such as copper, lead, or gold. Naturally occurring silver compounds, however, are the primary sources of silver in the environment. The most abundant naturally occurring silver compound is silver sulfide [21548- 73-2] (argentite), Ag2S, found alone and combined with iron, copper, and lead sulfides. Other naturally occur- ring silver compounds are silver sulfonantimonite [15983-65-0] (pyrargyrite), Ag3SbS3, silver arsenite [15122-57-3] (proustite), Ag3AsS3, silver selenide [1302-09-6], and silver telluride [12653-91-7]. Silver chloride (chlorargyrite) and silver iodide (iodargyrite) have also been found in substantial quantities in the western United States. Silver is a soft metal that preferentially forms lower oxidation states (þ1, þ2) and binds preferentially with sulfur (1). Studies show that silver levels in uncontaminated fine-grain sediments are typically similar to those for average crustal abundance, 0.05mg/g concentration of silver in fresh lake and river waters is typically<0.2mg/L, while those in contaminated estuary and harbor waters may be upward of from 5.1 to 33.9mg/g (2) produces a linear co-ordination of connecting bonds (3). It has one of the highest electronegativities resulting in strong covalent bonds. Its oxides are weakly held and are dissociate at about 2008C. -

Ethanol Denatured (Canadian)
GHS SAFETY DATA SHEET DENATURED ETHANOL _CANADIAN UN 3475 ONLY SDS DATE: 03/10/2021 SECTION 1: PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME (GHS Product Identifier): Denatured Ethanol (Canadian UN 3475 only) (Other means of Identification): Ethanol and Gasoline Mixture; Denatured Ethyl Alcohol PRODUCT INTENDED USE AND RESTRICTION: Canadian specification automobile fuel, fuel additive NAME, ADDRESS & TELEPHONE NUMBER OF THE RESPONSIBLE PARTY: Company Green Plains Trade Group LLC 1811 Aksarben Drive, Omaha, NE 68106 Phone: 402-884-8700 Email: [email protected] CHEMTREC PHONE (24HR Emergency Telephone): 1-800-424-9300 (Within U.S.A) INTERNATIONAL CHEMTREC CALL: 1-703-527-3887 OTHER CALLS: 1-402-884-8700 (M-F, 8 AM-5 PM, Central time (U.S.A & Canada); within U.S.A) FAX PHONE: 1-402-884-8776 (M-F, 8 AM-5 PM, Central time (U.S.A & Canada); within U.S.A) SECTION 1 NOTES: None Available SECTION 2: HAZARDS IDENTIFICATION GHS LABELING AND CLASSIFICATION: This product meets the definition of the following hazard classes as defined by the Globally Harmonized System of Classification and Labeling of Chemicals (GHS). GHS CLASSIFICATION ACCORDING TO ANNEX II: HEALTH ENVIRONMENTAL PHYSICAL Serious eye damage/eye irritation-2A Not classified Flammable Liquids-Category 2 Skin corrosion/irration-3 STOT SE-2 Acute toxicity (Oral)-Category 4 SIGNAL WORD: DANGER SYMBOL: HAZARD STATEMENT: H225: Highly flammable liquid and vapor. H319: Causes serious eye irritation. H315: Causes skin irritation. H335: May cause respiratory irritation H336: May cause drowsiness or dizziness H302: Harmful if swallowed. PREVENTIVE: P201: Obtain special instructions before use. P202: Do not handle until all safety precautions have been read and understood P210-Keep away from heat/sparks/open flames/hot surfaces.—No smoking.