A Cytophotometric Analysis of Anterior Pituitary Changes in Rats Exposed to Reduced Pressure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

Human General Histology
135 اووم څپرکي انډوکراين سيستم (Endocrine system) (hormones) (target cells) (receptors) autonomic (sinusoids) ductless glands thyroid gland Pineal gland Hypophysis cerebri(pituitary glands) supra renal( adrenal glands) parathyroid glands 136 اووم څپرکي انډوکراين سيسټم islets cell corpora lutea interstitial tissue (placenta) GIT amines neurotransmitters amines neuromodulator 137 اووم څپرکي انډوکراين سيسټم APUD cells neuroendocrine system system adrenaline, (amino acid derivatives) thyroxin noradrenalin thyroid vasopressin encephalin (small peptides) releasing hormone(TRH) TSH(thyroid stimulating parathormone hormone) cortisol Testosterone estrogen (steroids) 5,12,3 (Hypophysis Cerebri) (brain) pituitary gland (stalk) Ventricle infundibulum stalk pituitary fossa sphenoid pineal hypothalamus body 138 اووم څپرکي انډوکراين سيسټم Hypophysis cerebri Pars pars anterior pars nervosa pars posterior intermediate hypothalamus infundibulum infundibulum stalk )Pars posterior neurohypophysis median eminence (tuber cinereum) infundibulum pars neurohypophysis median eminence pars intermediate pars distalis anterior Adenohypophysis infundibulum pars anterior pars tuberalis adenohypophysis 139 اووم څپرکي انډوکراين سيسټم Adenohypophysis pars intermediate pars anterior adenohypophyisis Pars anterior fenestrated sinusoids (cords) chromophil chromophobic acidophil chromophil basophils orange G eosin PAS-positive hematoxylline Beta cells basophil Alpha cells Acidophil basophils acidophil (dese cored vesicles) alpha Beta Histochemical 140 اووم څپرکي انډوکراين -

© Copyright 2016 Wendy Yang
© Copyright 2016 Wendy Yang Role for cell-to-cell communication in stem cell specification toward pancreatic progenitors: relevance to the design of novel therapies for diabetes. Wendy Yang A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2016 Reading Committee: Vincenzo Cirulli, Chair Laura Crisa Paul D. Lampe Program Authorized to Offer Degree: Pharmacology University of Washington Abstract Role for cell-to-cell communication in stem cell specification toward pancreatic progenitors: relevance to the design of novel therapies for diabetes. Wendy Yang Chair of the Supervisory Committee: Vincenzo Cirulli Metabolism, Endocrinology & Nutrition Pancreatic islets of Langerhans, responsible for the production of hormones such as insulin and glucagon, develop from pluripotent pancreatic progenitors following their specification toward an endocrine phenotype. Based on the established role of cell-to-cell communication as an important mechanism regulating developmental decisions during embryonic life, I investigated the expression pattern of Connexins (Cxs), the building blocks of Gap Junction channels, in the developing human pancreas, and in an in vitro model of pancreatic progenitor differentiation from human embryonic stem cells (hESC). I also investigated the role of β1 integrins and an associated downstream effector, integrin-linked kinase (ILK), on islet development in mice. In a first series of experiments, I investigated the expression pattern of Cxs in the developing human pancreas. Results from these studies revealed that while Cx32 is predominantly expressed in the acinar tissue, Cx36 is primarily expressed in developing islet β- cells. Cx43 exhibited the most interesting expression pattern, being primarily detected in putative islet cell progenitors that delaminate from the pancreatic ductal epithelium and aggregate with developing islet cell clusters. -

The Physiology of Growth by S
Postgrad Med J: first published as 10.1136/pgmj.26.298.417 on 1 August 1950. Downloaded from 417 THE PHYSIOLOGY OF GROWTH BY S. LEONARD SIMPSON, M.A., M.D., F.R.C.P. Consultant Endocrinologist, St. Mary's Hospital, etc.; Formerly Research Wor7Aer, Lister Institute Although clinical and experimental evidence postulated an antagonism between growth and had previously indicated that skeletal growth sex-stimulating hormones. Further observations depended upon the pituitary gland, it was not suggested that corpora lutea were formed before until I92I that Evans and Long, at the University ovulation occurred, and that his emulsions con- of California, demonstrated that a saline emulsion tained gonadotrophic luteinizing hormone. Al- made from the anterior pituitary lobe of fresh though Evans' original giant rats were apparently glands of cattle contained a growth hormone. The adipose, later work (Lee and Schaffer, 1934), hypophyses were thrown into 40 per cent. ethyl showed that there was a retention of nitrogen and alcohol, agitated with a glass rod, transferred to a relative excess of body protein. This was also two changes of sterile normal saline, and triturated true of the excess of tissue deposited in and with force and speed with ocean sand, diluted with around the abdomen. Evans stated in a recent saline, and decanted. The layers of sand, cell discussion in London that rats treated with pure fragments and opaque pink fluid permitted the growth hormone, unlike those in the initial latter to be decanted. This emulsion, when experiments with crude extracts, were not injected daily in doses of I to i ml. -

Quantitation of Corticotrophs in the Pars Distalis of Stress-Prone Swine Beverly Ann Bedford Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1-1-1976 Quantitation of corticotrophs in the pars distalis of stress-prone swine Beverly Ann Bedford Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Veterinary Anatomy Commons Recommended Citation Bedford, Beverly Ann, "Quantitation of corticotrophs in the pars distalis of stress-prone swine" (1976). Retrospective Theses and Dissertations. 17953. https://lib.dr.iastate.edu/rtd/17953 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Quantitation of corticotrophs in the pars distalis of stress-prone swine by Beverly Ann Bedford A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degr~e of MASTER OF SCIENCE Department: Veterinary Anatomy, Pharmacology and Physiology Major: Veterinary Anatomy ., Signatures have been redacted for privacy ' I Iowa State University Ames, Iowa 1976 ii :E5 ll I q7(p ,g3r TABLE OF CONTENTS c,J. Page INTRODUCTION 1 LITERATURE REVIEW 4 Pituitary Gland 4 General morphology 4 Development 5 Blood supply 7 Staining techniques 7 Pars distalis 13 . Pars tuberalis 25 ·pars intermedia 28 Process of secretion 36 Neurohypophysis 41 Porcine Stress Syndrome 45 MATERIALS AND MET!iODS' 52 RESULTS 56 DISCUSSION 64 SUMMARY AND CONCLUSIONS 72 BIBLIOGRAPHY 73 ACKNOWLEDGMENTS 85 APPENDIX 86 1111408 1 INTRODUCTION As early as 1953, there came reports (Ludvigsen, 1953; Briskey et al., 1959) of pale soft exudative (PSE) post-mortem porcine muscu- lature which later stimulated research into the mechanisms responsible for this condition. -

Histology Histology
HISTOLOGY HISTOLOGY ОДЕСЬКИЙ НАЦІОНАЛЬНИЙ МЕДИЧНИЙ УНІВЕРСИТЕТ THE ODESSA NATIONAL MEDICAL UNIVERSITY Áiáëiîòåêà ñòóäåíòà-ìåäèêà Medical Student’s Library Серія заснована в 1999 р. на честь 100-річчя Одеського державного медичного університету (1900–2000 рр.) The series is initiated in 1999 to mark the Centenary of the Odessa State Medical University (1900–2000) 1 L. V. Arnautova O. A. Ulyantseva HISTÎLÎGY A course of lectures A manual Odessa The Odessa National Medical University 2011 UDC 616-018: 378.16 BBC 28.8я73 Series “Medical Student’s Library” Initiated in 1999 Authors: L. V. Arnautova, O. A. Ulyantseva Reviewers: Professor V. I. Shepitko, MD, the head of the Department of Histology, Cytology and Embryology of the Ukrainian Medical Stomatologic Academy Professor O. Yu. Shapovalova, MD, the head of the Department of Histology, Cytology and Embryology of the Crimean State Medical University named after S. I. Georgiyevsky It is published according to the decision of the Central Coordinational Methodical Committee of the Odessa National Medical University Proceedings N1 from 22.09.2010 Навчальний посібник містить лекції з гістології, цитології та ембріології у відповідності до програми. Викладено матеріали теоретичного курсу по всіх темах загальної та спеціальної гістології та ембріології. Посібник призначений для підготовки студентів до практичних занять та ліцензійного екзамену “Крок-1”. Arnautova L. V. Histology. A course of lectures : a manual / L. V. Arnautova, O. A. Ulyantseva. — Оdessa : The Оdessa National Medical University, 2010. — 336 p. — (Series “Medical Student’s Library”). ISBN 978-966-443-034-7 The manual contains the lecture course on histology, cytology and embryol- ogy in correspondence with the program. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria. -

Observations on the Histology and Ultrastructure
OBSERVATIONS ON THE HISTOLOGY AND ULTRASTRUCTURE OF THE PARS DISTALIS OF THE RABBIT HYPOPHYSIS IN ORGAN CULTURE A Thesis submitted for the degree of Doctor of Philosophy in the University of London by SNEHLATA PATHAK Department of Cellular Biology 1970 and Histology, St. Mary's Hospital Medical School, London W.2. ABSTRACT The histology and ultrastrueture of the pars distalis of the rabbit hypophysis was studied after different periods of organ culture, and the best technique for the maintenance of the maximum proportion of the explant was assessed by comparing cultures grown in different conditions. Explants in air with a medium buffered. with N.2-hydroxyethylpiperazine-N1-2- ethanesulphonic acid (HEPES), not previously used in organ culture, proved more satisfactory than explants in carbogen with bicarbonate buffered 199, and cultures were maintained for more than 3 weeks. Material from young animals survived better than from old. The survival of cells was assessed on the basis of their cytological integrity when explants were examined by light microscopy after specific staining and by electron microscopy; DNA and RNA fluorescence with acridine orange was a valuable indicator. Also, cell multiplication was identified by direct observation of mitosis, by the application of the colchicine technique and by autoradiography. During culture, prolactin cells showed physiological signs of secretion (demonstrated by combined culture with mammary gland) and morphological signs of an increase of secretory activity. Morphological signs of reduced secretory activity appeared in the presence of hypothalamic tissue (combined culture) or extract. Somatotrophs and gonadotrophs. showed signs of low-level secretory activity in solitary pars distalis culture and of increased activity in combined culture with hypothalamus. -

Involvement of Gap Junctional Communication in Secretion
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Biochimica et Biophysica Acta 1719 (2005) 82 – 101 http://www.elsevier.com/locate/bba Review Involvement of gap junctional communication in secretion Laetitia Michon 1, Rachel Nlend Nlend 1, Sabine Bavamian, Lorraine Bischoff, Nathalie Boucard, Dorothe´e Caille, Jose´ Cancela, Anne Charollais, Eric Charpantier, Philippe Klee, Manon Peyrou, Ce´line Populaire, Laurence Zulianello, Paolo Meda * Department of Cell Physiology and Metabolism, University of Geneva, C.M.U., 1 rue Michel Servet, 1211 Geneva 4, Switzerland Received 11 July 2005; received in revised form 31 October 2005; accepted 7 November 2005 Available online 18 November 2005 Abstract Glands were the first type of tissues in which the permissive role of gap junctions in the cell-to-cell transfer of membrane-impermeant molecules was shown. During the 40 years that have followed this seminal finding, gap junctions have been documented in all types of multicellular secretory systems, whether of the exocrine, endocrine or pheromonal nature. Also, compelling evidence now indicates that gap junction-mediated coupling, and/or the connexin proteins per se, play significant regulatory roles in various aspects of gland functions, ranging from the biosynthesis, storage and release of a variety of secretory products, to the control of the growth and differentiation of secretory cells, and to the regulation of gland morphogenesis. This review summarizes this evidence in the light of recent reports. D 2005 Elsevier B.V. All rights reserved. Keywords: Exocrine gland; Endocrine gland; Enzyme; Hormone; Ca2+; Synchronization Contents 1. -
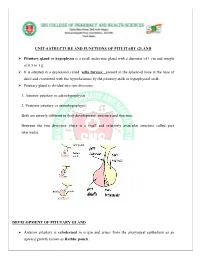
Unit-4 Structure and Functions of Pituitary Gland
UNIT-4 STRUCTURE AND FUNCTIONS OF PITUITARY GLAND Pituitary gland or hypophysis is a small endocrine gland with a diameter of 1 cm and weight of 0.5 to 1 g. It is situated in a depression called ‘sella turcica’, present in the sphenoid bone at the base of skull and connected with the hypothalamus by the pituitary stalk or hypophyseal stalk. Pituitary gland is divided into two divisions: 1. Anterior pituitary or adenohypophysis 2. Posterior pituitary or neurohypophysis. Both are entirely different in their development, structure and function. Between the two divisions, there is a small and relatively avascular structure called pars intermedia. DEVELOPMENT OF PITUTARY GLAND Anterior pituitary is ectodermal in origin and arises from the pharyngeal epithelium as an upward growth known as Rathke pouch. Posterior pituitary is neuroectodermal in origin and arises from hypothalamus as a downward diverticulum. Rathke pouch and the downward diverticulum from hypothalamus grow towards each other and meet in the midway between the roof of the buccal cavity and base of brain. There, the two structures lie close together. ANTERIOR PITUITARY OR ADENOHYPOPHYSIS Anterior pituitary is also known as the master gland because it regulates many other endocrine glands through its hormones. Anterior pituitary consists of three parts 1. Pars distalis 2. Pars tuberalis 3. Pars intermedia. Anterior pituitary has two types of cells 1. Chromophobe cells 2. Chromophil cells. Chromophobe Cells Chromophobe cells are not secretory in nature, but are the precursors of chromophil cells. Chromophil Cells On the basis of secretory nature chromophil cells are classified into five types: i. -

Light and Electron Microscopic Observations on the Anterior Pituitary of the Mouse Injected with Dl-Thyroxine By
Okajimas Fol. anat. jap., 43: 21-51, 1967 Light and Electron Microscopic Observations on the Anterior Pituitary of the Mouse injected with dl-Thyroxine By Tomiji Uchida Department of Anatomy, Nagoya University School of Medicine, Nagoya, Japan (Director : Prof. Dr. Ka z u m a r o Y a m ad a) Introduction Probably, the first suggestion of the existence of a functional relationship between the thyroid and hypophysis was given by Niepce (1851) who described pituitary enlargement in a series of goitrous cretins. Since then numerous studies have provided detailed evidence of this relationship. The reciprocal interrelationship be- tween thyroid stimulating hormone (TSH) secretion in the anterior hypophysis and the circulating levels of thyroid hormone has been well established, and in general, procedures which reduce effective plasma concentrations of thyroid hormone increase thyrotrophic hormone release in the anterior pituitary, whereas increased circulat- ing levels of thyroxine inhibit thyrotophic hormone secretion. This negative feedback mechanism resides both in the level of the pituitary and of a hypothalamic " TSH releasing center " (S o 1 o m on and Dowling, '60). Earlier views (Ma rin e, Rosen and Spar k, '35; Morris, '52) favored pituitary acidophile cell as the source of thyrotrophic hormone, but results of many recent studies implicate the basophile cell (Zeckwer, '38a and '38b; Griesbach and Purves, '45 Pur v es and Griesbac h, '46a, '46b, '51a, '51b, '51c, '57a and '57b; Goldberg and Chaikoff, '50; Salter, '50, Halmi, '50, '51, '52a , '52b, and 52c ; R ennel s, '53; Halm i and G u d e, '54 D'Angelo, '53 and '55; Knigge, '55; Elf tman, '58; Mura - s h i m a, '60 and others). -

Endocrine System Hormonal Regulation Endocrine Glands
Endocrine system Hormonal regulation Endocrine glands • Glands w/o ducts • Secretory cells release their products, hormones, into the extracellular space and blood stream • Alternatively, the hormones may affect neighbor cells (paracrine) • Structure: • c.t. – capsule + septs • irregular clumps or cords of the cells • network of capillaries • fenestrated capillaries • sinusoids Hypophysis – pituitary gland Pituitary gland – anterior pituitary Pituitary gland – anterior pituitary Chromophil cells - Acidophilic cells (produce proteins) somatotrophs mammotrophs (or lactotrophs) - Basophilic cells (produce glycoproteins) thyrotrophs produce thyroid stimulating hormone (TSH or thyrotropin). gonadotrophs produce follicle stimulating hormone (FSH) and luteinizing hormone (LH) corticotrophs (or adrenocorticolipotrophs) Chromophobe cells Adenohypophysis Adenohypophysis oranž G + PAS Neurohypophysis x Adenohypophysis Neurohypophysis - structure Structure unmyelinated nerve fibres derived from neurosecretory cells of the supraoptic and paraventricular hypothalamic nuclei pituicytes /neuroglia/ Function Two hormones are oxytocin, which stimulates the contraction of smooth muscle cell in the uterus and participates in the milk ejection reflex, and antidiuretic hormone (ADH or vasopressin), which facilitates the concentration of urine in the kidneys and, thereby, the retention of water. Usually only the oval or round nuclei of the pituicytes are visible. Hypothalamic nerve fibres typically terminate close to capillaries. Scattered, large