The Corticospinal Tract from the Viewpoint of Brain Rehabilitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
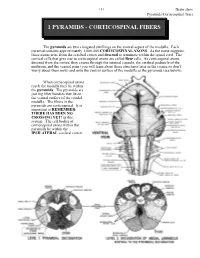
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or -

Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) There are multiple motor neuron diseases. Each has its own defining features and many characteristics that are shared by all of them: Degenerative disease of the nervous system Progressive despite treatments and therapies Begins quietly after a period of normal nervous system function ALS is the most common motor neuron disease. One of its defining features is that it is a motor neuron disease that affects both upper and lower motor neurons. Anatomical Involvement ALS is a disease that causes muscle atrophy in the muscles of the extremities, trunk, mouth and face. In some instances mood and memory function are also affected. The disease operates by attacking the motor neurons located in the central nervous system which direct voluntary muscle function. The impulses that control the muscle function originate with the upper motor neurons in the brain and continue along efferent (descending) CNS pathways through the brainstem into the spinal cord. The disease does not affect the sensory or autonomic system because ALS affects only the motor systems. ALS is a disease of both upper and lower motor neurons and is diagnosed in part through the use of NCS/EMG which evaluates lower motor neuron function. All motor neurons are upper motor neurons so long as they are encased in the brain or spinal cord. Once the neuron exits the spinal cord, it operates as a lower motor neuron. 1 Upper Motor Neurons The upper motor neurons are derived from corticospinal and corticobulbar fibers that originate in the brain’s primary motor cortex. They are responsible for carrying impulses for voluntary motor activity from the cerebral cortex to the lower motor neurons. -

Topographic Organization of Corticospinal Projections from the Frontal Lobe: Motor Areas on the Lateral Surface of the Hemisphere
The Journal of Neuroscience, March 1993, 133): 952-980 Topographic Organization of Corticospinal Projections from the Frontal Lobe: Motor Areas on the Lateral Surface of the Hemisphere San-Qiang He, Richard P. Dum, and Peter L. Strick Research Service, Veterans Affairs Medical Center, and Departments of Neurosurgery and Physiology, SUNY Health Science Center at Syracuse, Syracuse, New York 13210 We examined the topographic organization of corticospinal PMd that project most densely to upper cervical segments neurons in the primary motor cortex and in the two premotor were largely separate from those that project most dense/y areas on the lateral surface of the hemisphere [i.e., the dor- to lower cervical segments. Furthermore, we found two sep- sal premotor area (PMd) and the ventral premotor area (PMv)]. arate regions within area 4 that send corticospinal projec- In two macaques, we labeled corticospinal neurons that pro- tions primarily to the lower cervical segments. One of these ject beyond T7 or S2 by placing crystals of HRP into the regions was located within the classical “hand” area of the dorsolateral funiculus at these segmental levels. In another primary motor cortex. The other was located at the medial seven macaques, we labeled corticospinal neurons that pro- edge of arm representation in the primary motor cortex. ject to specific segmental levels of the spinal cord by in- These results provide new insights into the pattern of body jecting the fluorescent tracers fast blue and diamidino yellow representation in the primary motor cortex, PMd, and PMv. into the gray matter of the cervical and lumbosacral seg- Our findings support the classic distinction between the ments. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

How Does the Brain Produce Movement?
p CHAPTER 10 How Does the Brain Produce Movement? The Hierarchical Control The Basal Ganglia and of Movement the Cerebellum The Forebrain and Movement Initiation The Basal Ganglia and Movement Force The Brainstem and Species-Typical Movement Focus on Disorders: Tourette’s Syndrome Focus on Disorders: Autism The Cerebellum and Movement Skill The Spinal Cord and Movement Execution Focus on Disorders: Paraplegia The Organization of the Somatosensory System The Organization of the Somatosensory Receptors and Sensory Perception Motor System Dorsal-Root Ganglion Neurons The Motor Cortex The Somatosensory Pathways to the Brain The Corticospinal Tracts Spinal-Cord Responses to Somatosensory Input The Motor Neurons The Vestibular System and Balance The Control of Muscles Exploring the Somatosensory The Motor Cortex and Skilled System Movements The Somatosensory Homunculus Investigating Neural Control of Skilled Movements The Effects of Damage to the Somatosensory Cortex The Control of Skilled Movements in Other Species The Somatosensory Cortex and Complex How Motor Cortex Damage Affects Skilled Movement Movements Kevork Djansezian/AP Photo Micrograph: Dr. David Scott/Phototake 354 I p amala is a female Indian elephant that lives at the In one way, however, Kamala uses this versatile trunk zoo in Calgary, Canada. Her trunk, which is really very unusually for an elephant (Onodera & Hicks, 1999). K just a greatly extended upper lip and nose, con- She is one of only a few elephants in the world that paints sists of about 2000 fused muscles. A pair of nostrils runs its with its trunk (Figure 10-1). Like many artists, she paints length and fingerlike projections are located at its tip. -

Corticospinal Tract (CST) Reconstruction Based on Fiber
Corticospinal Tract (CST) reconstruction based on fiber orientation distributions (FODs) tractography Youshan Zhang Computer Science and Engineering Department Lehigh University Bethlehem, PA [email protected] Abstract—The Corticospinal Tract (CST) is a part of pyra- About 75%-90% of the CST fibers descend to the lateral midal tract (PT) and it can innervate the voluntary movement corticospinal tract by decussation of the pyramid, but a few of skeletal muscle through spinal interneurons (the 4th layer of fibers form the anterior corticospinal tract by not crossing the Rexed gray board layers), and anterior horn motorneurons (which control trunk and proximal limb muscles). Spinal cord the pyramid [9], [10]. The fibers, which first passed through injury (SCI) is a highly disabling disease often caused by the medial posterior spinocerebellar tract then went into traffic accidents. The recovery of CST and the functional the lumbosacral spinocerebellar tract, were not yet appeared reconstruction of spinal anterior horn motor neurons play an [11], [12]. The main function of the CST is innervating essential role in the treatment of SCI. However, the localization the voluntary movement of skeletal muscle through spinal and reconstruction of CST are still challenging issues, the accuracy of the geometric reconstruction can directly affect interneurons (the 4th layer of the Rexed gray board layers), the results of the surgery. The main contribution of this paper and anterior horn motorneurons (which control trunk and is the reconstruction of the CST based on the fiber orientation proximal limb muscles), then the CST terminates in the spinal distributions (FODs) tractography. Differing from tensor-based motor cells (which control the fine motor of small muscle tractography in which the primary direction is a determined in extremities) [13]. -

Quiz 42, Understanding Motor Systemssr
M O T O R Pathways SNACC QUIZ #42 Quiz #42 UNDERSTANDING MOTOR PATHWAYS IN THE CENTRAL NERVOUS SYSTEM M. ANGELE THEARD, MD ANESTHESIOLOGIST, LEGACY EMANUEL HOSPITAL, PORTLAND OREGON QUIZ TEAM: SHOBANA RAJAN, MD SUNEETA GOLLAPUDY, MD VERGHESE CHERIAN, MD THIS QUIZ IS PUBLISHED ON BEHALF OF THE EDUCATION COMMITTEE OF SNACC To Q 1 1. MOTOR SYSTEMS IN HUMANS INCLUDE ALL OF THE FOLLOWING EXCEPT: A. Corticospinal tract B. Corticobulbar tract C. Extrapyramidal system D. Spinothalamic tract To Q 2 A. CORTICOSPINAL TRACT This is true. Axons from the corticospinal tract (CST)or pyramidal tract carry information from the precentral gyrus (brodmann area 4 of the motor cortex), the supplemental, and premotor cortices (area 6) to Lower motor neurons (LMNs) which will synapse with muscle cells in the body effecting voluntary movement. While most of the fibers from this tract are from the motor cortex, some fibers originate from the primary sensory area of the brain. Felten et al, Netter’s Atlas of Neuroscience 2nd ed. Philadelphia: Elsevier Saunders, 2010:357-86 B. CORTICOBULBAR TRACT This is true. Like the CST, axons from this descending tract originate in the motor cortex and enter the brainstem synapsing on the LMNs of cranial nerves. This tract runs alongside the CST passing through the internal capsule and into the medulla oblongata (also called bulbar) before synapsing with the LMNs of cranial nerves (CN). The muscles of the face, head and neck are controlled by the corticobulbar system. Felten et al, Netter’s Atlas of Neuroscience 2nd ed. Philadelphia: Elsevier Saunders, 2010:357-86 Liebman, et al. -

Functions of the Corticospinal and Corticobulbar Tracts in the Human Newborn
Journal of Pediatric Neurology 2003; 1(1): 3-8 www.jpneurology.org REVIEW ARTICLE Functions of the corticospinal and corticobulbar tracts in the human newborn Harvey B. Sarnat Departments of Pediatrics and Pathology, David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center, Los Angeles, California, U.S.A. Abstract adduction (“cortical thumb”) and fisting), and poor suck and swallow in affected neonates, The corticospinal and corticobulbar tracts regardless of the cause of cerebral cortical (CST, CBT) are immature at birth, in impairment or its reversibility or irreversibility. neuroanatomical terms of myelination and In summary, the CST and CBT are indeed terminal axonal sprouting for multiple synaptic important functional pathways in the neonate, contact; these developmental features are not but many of their functions are very different mature until 2 years of age. Physiologically, the than in the adult. (J Pediatr Neurol 2003; 1(1): CST is mainly inhibitory. Nevertheless, these 3-8). pathways have an important role to play in normal neurological function at this age, though Key words: corticospinal tract, muscle tone, neonate, different from their functions in the older child posture, stretch reflexes, suck, swallow, tactile reflexes. and adult. They are important in the neonate by 1) inhibition of the monosynaptic stretch reflexes at spinal cord levels, beginning at 25 weeks gestation Introduction or earlier; 2) influence upon muscle tone, hence posture, by mediating proximal flexion in axial It has long been debated in neonatal neurology and limb girdle muscles and distal extension of whether the corticospinal tract (CST) plays a role the fingers and toes and abduction of the thumbs; in the neurological function of the premature and 3) antagonism of the proximal extension and term neonate, or whether the newborn essentially distal flexion and adduction from the medial functions reflexively at a brainstem level. -

The Clinicoanatomic Uniqueness of the Human Pyramidal Tract And
de Oliveira-Souza R. J Neurol Neuromedicine (2017) 2(2): 1-5 Neuromedicine www.jneurology.com www.jneurology.com Journal of Neurology & Neuromedicine Mini Review Article Open Access The Clinicoanatomic Uniqueness of the Human Pyramidal Tract and Syndrome Ricardo de Oliveira-Souza D’Or Institute for Research & Education (IDOR) and Federal University of the State of Rio de Janeiro, Brazil ABSTRACT Article Info The chief goal of the present review is to present clinicoanatomic evidence Article Notes Received: November 28, 2016 that, (i) in contrast to most vertebrates, spastic hemiplegia in man is a symptom of Accepted: February 02, 2017 damage to the pyramidal tracts, and (ii) although extrapyramidal structures are often injured as a contingency of anatomical proximity in cases of pyramidal damage, the *Correspondence: extrapyramidal system plays no role in the production of human spastic hemiplegia. Ricardo de Oliveira-Souza The views herein discussed reconcile several apparent incongruences concerning Rua Diniz Cordeiro, 30/2º andar the pathophysiology of the human pyramidal syndrome. From a neurobiological Rio de Janeiro, RJ, Brazil Email: [email protected] perspective, the progressive commitment to occasional, habitual and obligate bipedalism fostered a profound internal reorganization of the mammalian brain © 2017 de Oliveira-Souza R. This article is distributed under at the early stages of human phylogenesis. The major anatomical counterpart of the terms of the Creative Commons Attribution 4.0 International this reorganization was an unprecedented increase of the ansa lenticularis fiber License system, which ultimately redirected the product of subcortical motor activity up Keywords to the motor cortices from which the pyramidal tracts originate. -

Both Pyramidal Tracts and Extrapyramidal Both Starts from Cortex
Motor tracts Both pyramidal tracts and extrapyramidal both starts from cortex: Area 4 Area 6 Area 312 Pyramidal: mainly from area 4 Extrapyramidal: mainly from area 6 area 6 Premotor area: uses external cues Suplemantary motor area: uses internal cues Lateral corticospinal tract The upper motor neurons of these tracts originate in the precentral gyrus of the cerebral cortex In midbrain:middle three- fifths of the basis pedunculi of the midbrain In medulla oblongata: pyramids Most of the fibers (85 percent) cross over (decussate) to the opposite side in the pyramidal decussation, where they continue to descend in the lateral funiculus of the spinal cord as the lateral corticospinal tract (LCST). The tract descends all the way of spinal cord with fibers continually leaving it in order to synapse on interneurons in the anterior gray horn. ( Some even synapse directly on alpha and gamma motor neurons) Those corticospinal fibers which do not decussate in the medulla continue descending on the same (ipsilateral) side of the cord and become the anterior corticospinal tract (ACST). corticospinal tract for fine skilled movements Lateral corticospinal tract descends the full length of the spinal cord LCST fibers synapse with alpha and gamma nuclei of the Cervical region (55%) (great effect on the upper limb) Thoracic 20% Lumbar and Sacral 25% The lateral corticospinal tract synapses mainly by interneurons In lamine IV, V, VI, VII, VIII Exception: 3% originate from the fifth layer of area 4 (giant cells of betz) synapse directly. (Accurate movements) The anterior corticospinal tract acts on the proximal muscles of upper limb (shoulder muscle) of the ipsilateral and contralateral sides Fibers leave the tract at various levels to cross over in the anterior white commissure to synapse on interneurons in the anterior gray horn. -

Pure Motor Hemiplegia, Medullary Pyramid Lesion, and Olivary Hypertrophy
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.9.877 on 1 September 1976. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1976, 39, 877-884 Pure motor hemiplegia, medullary pyramid lesion, and olivary hypertrophy J. E. LEESTMA' AND A. NORONHA From the Departments ofPathology and Neurology, Northwestern University School ofMedicine, Chicago, Illinois, USA SYNOPSIS The case is presented of a 60 years old man who developed sudden right hemiplegia without other accompanying neurological signs and later a spastic hemiparesis. Neuropathological studies indicated an ischaemic lesion ofthe left medullary pyramid which was accompanied by hyper- trophy of the left inferior olivary nucleus. An additional lesion, demyelination of the right gracile tract, is poorly explained. This case represents the second reported instance of pure motor hemiplegia due to a circumscribed lesion in the medullary pyramid and possibly an unique instance of olivary hypertrophy without obvious damage to the central tegmental tract, ipsilateral superior cerebellar guest. Protected by copyright. peduncle, or contralateral dentate nucleus. The olivary hypertrophy is thought to have arisen from local damage to the termination ofthe central tegmental fibres at the left inferior olivary nucleus. The question of the development of spasticity in a pure pyramidal tract lesion is discussed. Fisher and Curry (1965) defined pure motor consciousness, headache, convulsion, paraesthesiae, hemiplegia as a paralysis, complete or incomplete, vertigo, diplopia, dysphagia, or visual difficulty. One of the face, arm, and leg on one side unaccom- month before this episode hypertension was noted for panied by sensory signs, visual field defect, the first time. He was in congestive heart failure and was treated with methyldopa, digoxin and frusemide.