The Origin of Corticospinal Projections from the Premotor Areas in the Frontal Lobe
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -
Lateralization of Function Human Brain
Lateralization of Function Dr. Coulson Cognitive Science Department UCSD Human Brain • An extension of the spinal cord 1 Cerebral Hemispheres Corpus Callosum 2 Cartoon View of Brain Cerebral Lobes 3 Neurons • Brain composed of neurons – 100 billion • Neurons both send and receive signals to other cells in form of pulses • Important parts – Cell body –Axon – Synapse Connectivity • Each neuron connected to 10,000 other neurons • Point of contact is the synapse • Computing power of brain comes from connections 4 Cortex • Two millimeters thick and has area of 1.5 square meters Cartoon View: Frontal Lobe • In front of central sulcus • Decisions, judgments, emotions 5 Cartoon View: Parietal Lobe • Behind central sulcus • Perception of stimuli related to touch, pressure, temperature, pain Cartoon View: Temporal Lobe • Below lateral fissure • Perception, recognition, auditory processing 6 Cartoon View: Occipital Lobe • Located at back of brain, behind the parietal lobe and temporal lobe • Vision Lateralization of Function • One side of the brain is more crucial for a given function and/or more efficient at the underlying computational tasks • Typically a matter of degree – Strongly vs. Weakly Lateralized • Motor control a good example of a lateralized function 7 Sensorimotor Cortex 8 Motor Control What about language? • Language is a paradigmatic example of a lateralized cognitive phenomenon 9 Wada Test Lateralization of Function • Most evidence of lateralized brain function comes from observing how brain damage affects behavior on various sorts -

Prefrontal and Posterior Parietal Contributions to the Perceptual Awareness of Touch M
www.nature.com/scientificreports OPEN Prefrontal and posterior parietal contributions to the perceptual awareness of touch M. Rullmann1,2,5, S. Preusser1,5 & B. Pleger1,3,4* Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identifed motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated efects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present fndings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes. Te primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1–3. -
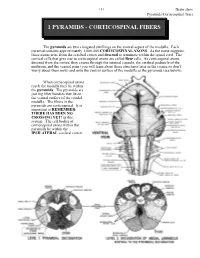
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography
3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography Julien Dauguet1, Sharon Peled2, Vladimir Berezovskii3, Thierry Delzescaux4, Simon K. Warfield1, Richard Born3, and Carl-Fredrik Westin5 1 Computational Radiology Laboratory, Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2 Harvard Center for Neurodegeneration and Repair, Boston, USA 3 Department of Neurobiology, Harvard Medical School, Boston, USA 4 Service Hospitalier Fr´ed´eric Joliot, CEA, Orsay, France 5 Laboratory of Mathematics in Imaging, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA Abstract. A classical neural tract tracer, WGA-HRP, was injected at multiple sites within the brain of a macaque monkey. Histological sections of the labeled fiber tracts were reconstructed in 3D, and the fibers were segmented and registered with the anatomical post-mortem MRI from the same animal. Fiber tracing along the same pathways was performed on the DTI data using a classical diffusion tracing technique. The fibers derived from the DTI were compared with those segmented from the histology in order to evaluate the performance of DTI fiber tracing. While there was generally good agreement between the two methods, our results reveal certain limitations of DTI tractography, particularly at regions of fiber tract crossing or bifurcation. 1 Introduction Tracing neural connections lies at the heart of neuroanatomy, and has profound implications for the study of neural function and for the study of developmental and adult plasticity of the nervous system. It is also important for the study of neurodegenerative diseases, and for the planning of neurosurgical procedures, such as tumor ablation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

Function of Cerebral Cortex
FUNCTION OF CEREBRAL CORTEX Course: Neuropsychology CC-6 (M.A PSYCHOLOGY SEM II); Unit I By Dr. Priyanka Kumari Assistant Professor Institute of Psychological Research and Service Patna University Contact No.7654991023; E-mail- [email protected] The cerebral cortex—the thin outer covering of the brain-is the part of the brain responsible for our ability to reason, plan, remember, and imagine. Cerebral Cortex accounts for our impressive capacity to process and transform information. The cerebral cortex is only about one-eighth of an inch thick, but it contains billions of neurons, each connected to thousands of others. The predominance of cell bodies gives the cortex a brownish gray colour. Because of its appearance, the cortex is often referred to as gray matter. Beneath the cortex are myelin-sheathed axons connecting the neurons of the cortex with those of other parts of the brain. The large concentrations of myelin make this tissue look whitish and opaque, and hence it is often referred to as white matter. The cortex is divided into two nearly symmetrical halves, the cerebral hemispheres . Thus, many of the structures of the cerebral cortex appear in both the left and right cerebral hemispheres. The two hemispheres appear to be somewhat specialized in the functions they perform. The cerebral hemispheres are folded into many ridges and grooves, which greatly increase their surface area. Each hemisphere is usually described, on the basis of the largest of these grooves or fissures, as being divided into four distinct regions or lobes. The four lobes are: • Frontal, • Parietal, • Occipital, and • Temporal. -

A Core Speech Circuit Between Primary Motor, Somatosensory, and Auditory Cortex
bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 1 A core speech circuit between primary motor, somatosensory, and auditory cortex: Evidence from connectivity and genetic descriptions * ^ Jeremy I Skipper and Uri Hasson * Experimental Psychology, University College London, UK ^ Center for Mind/Brain Sciences (CIMeC), University of Trento, Italy, and Center for Practical Wisdom, Dept. of Psychology, The University of Chicago Running title: Speech core Words count: 20,362 Address correspondence to: Jeremy I Skipper University College London Experimental Psychology 26 Bedford Way London WC1H OAP United Kingdom E-mail: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/139550; this version posted May 19, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Speech core 2 Abstract What adaptations allow humans to produce and perceive speech so effortlessly? We show that speech is supported by a largely undocumented core of structural and functional connectivity between the central sulcus (CS or primary motor and somatosensory cortex) and the transverse temporal gyrus (TTG or primary auditory cortex). Anatomically, we show that CS and TTG cortical thickness covary across individuals and that they are connected by white matter tracts. -

The Prefrontal Cortex
Avens Publishing Group Inviting Innovations Open Access Review Article J Hum Anat Physiol July 2017 Volume:1, Issue:1 © All rights are reserved by Ogeturk. AvensJournal Publishing of Group Inviting Innovations Human Anatomy The Prefrontal Cortex: A Basic & Physiology Embryological, Histological, Anatomical, and Functional Ramazan Fazıl Akkoc and Murat Ogeturk* Department of Anatomy, Firat University, Turkey Guideline *Address for Correspondence Murat Ogeturk, Firat University, Faculty of Medicine, 23119 Elazig, Turkey, Tel: +90-424-2370000 (ext: 4654); Fax: +90-424-2379138; Keywords: Prefrontal cortex; Working memory; Frontal lobe E-Mail: [email protected] Abstract Submission: 24 May, 2017 Accepted: 11 July, 2017 The prefrontal cortex (PFC) unites, processes and controls the Published: 19 August, 2017 information coming from cortex and subcortical structures, and Copyright: © 2017 Akkoc RF. This is an open access article distributed decides and executes goal-oriented behavior. A major function of PFC under the Creative Commons Attribution License, which permits is to maintain the attention. Furthermore, it has many other functions unrestricted use, distribution, and reproduction in any medium, provided including working memory, problem solving, graciousness, memory, the original work is properly cited. and intellectuality. PFC is well developed in humans and localized to the anterior of the frontal lobe. This article presents a systematic review and detailed summary of embryology, histology, anatomy, functions and lesions of PFC. I. Lamina zonalis: Contains few Cajal horizontal cells. The axons of Martinotti cells located at deep layers, the last branches of the apical dendrites of pyramidal cells, and the last branches Introduction of the afferent nerve fibers extend to this lamina. -

Neural Correlates Underlying Change in State Self-Esteem Hiroaki Kawamichi 1,2,3, Sho K
www.nature.com/scientificreports OPEN Neural correlates underlying change in state self-esteem Hiroaki Kawamichi 1,2,3, Sho K. Sugawara2,4,5, Yuki H. Hamano2,5,6, Ryo Kitada 2,7, Eri Nakagawa2, Takanori Kochiyama8 & Norihiro Sadato 2,5 Received: 21 July 2017 State self-esteem, the momentary feeling of self-worth, functions as a sociometer involved in Accepted: 11 January 2018 maintenance of interpersonal relations. How others’ appraisal is subjectively interpreted to change Published: xx xx xxxx state self-esteem is unknown, and the neural underpinnings of this process remain to be elucidated. We hypothesized that changes in state self-esteem are represented by the mentalizing network, which is modulated by interactions with regions involved in the subjective interpretation of others’ appraisal. To test this hypothesis, we conducted task-based and resting-state fMRI. Participants were repeatedly presented with their reputations, and then rated their pleasantness and reported their state self- esteem. To evaluate the individual sensitivity of the change in state self-esteem based on pleasantness (i.e., the subjective interpretation of reputation), we calculated evaluation sensitivity as the rate of change in state self-esteem per unit pleasantness. Evaluation sensitivity varied across participants, and was positively correlated with precuneus activity evoked by reputation rating. Resting-state fMRI revealed that evaluation sensitivity was positively correlated with functional connectivity of the precuneus with areas activated by negative reputation, but negatively correlated with areas activated by positive reputation. Thus, the precuneus, as the part of the mentalizing system, serves as a gateway for translating the subjective interpretation of reputation into state self-esteem.