Transneuronal Downregulation of the Premotor Cholinergic System After Corticospinal Tract Loss
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Individual Neuronal Subtypes Control Initial Myelin Sheath Growth and Stabilization Heather N
Nelson et al. Neural Development (2020) 15:12 https://doi.org/10.1186/s13064-020-00149-3 RESEARCH ARTICLE Open Access Individual neuronal subtypes control initial myelin sheath growth and stabilization Heather N. Nelson, Anthony J. Treichel, Erin N. Eggum, Madeline R. Martell, Amanda J. Kaiser, Allie G. Trudel, James R. Gronseth, Samantha T. Maas, Silas Bergen and Jacob H. Hines* Abstract Background: In the developing central nervous system, pre-myelinating oligodendrocytes sample candidate nerve axons by extending and retracting process extensions. Some contacts stabilize, leading to the initiation of axon wrapping, nascent myelin sheath formation, concentric wrapping and sheath elongation, and sheath stabilization or pruning by oligodendrocytes. Although axonal signals influence the overall process of myelination, the precise oligodendrocyte behaviors that require signaling from axons are not completely understood. In this study, we investigated whether oligodendrocyte behaviors during the early events of myelination are mediated by an oligodendrocyte-intrinsic myelination program or are over-ridden by axonal factors. Methods: To address this, we utilized in vivo time-lapse imaging in embryonic and larval zebrafish spinal cord during the initial hours and days of axon wrapping and myelination. Transgenic reporter lines marked individual axon subtypes or oligodendrocyte membranes. Results: In the larval zebrafish spinal cord, individual axon subtypes supported distinct nascent sheath growth rates and stabilization frequencies. Oligodendrocytes ensheathed individual axon subtypes at different rates during a two-day period after initial axon wrapping. When descending reticulospinal axons were ablated, local spinal axons supported a constant ensheathment rate despite the increased ratio of oligodendrocytes to target axons. Conclusion: We conclude that properties of individual axon subtypes instruct oligodendrocyte behaviors during initial stages of myelination by differentially controlling nascent sheath growth and stabilization. -

Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Cytation C10 Confocal Imaging Reader
Application Bulletin Whole-Organism Imaging of Spinal Motor Neurons and Musculature with the Cytation C10 Confocal Imaging Reader Introduction Zebrafish have long held an essential oler in developmental biology research due to their many and often cited advantages, both biological and practical. Recently, zebrafish amenability to genome editing techniques such as CRISPR, high-throughput screening, and predictive value in drug discovery toxicol- ogy studies1, combined with their exceptional accessibility to optogenetic manipulation and live-imaging techniques highlight the essential place zebrafish have in the toolkit of health-driven research. Indeed, zebrafish are lowering the barrier toin vivo research of devastating neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS, also known as Lou Gehrig’s disease)2, as their complete spinal neuron and muscle unit organization is entirely accessible to observation through light microscopy. This application bulletin demonstrates the capability of whole-organism imaging on the newly intro- duced BioTek Cytation C10 confocal imaging reader, with a focus on spinal motor neurons, neuromus- cular synapses, and musculature of the zebrafish model organism. The optical sectioning of the spin- ning disk confocal system is essential to resolving the deep tissue structures, such as the fine neuronal processes, and provides a literal clearer view of subcellular details when imaging thick samples. Materials and Methods Key Words: Unless otherwise noted, all chemicals were obtained from Sigma Aldrich (St. Louis, MO). General main- tenance of zebrafish followed established methods3. Wild-type male and female Danio rerio were main- Confocal Microscopy tained at 28 °C on a 14/10 hour light/dark cycle. Adults were crossed and eggs promptly collected in Optical Sectioning EM3 media4. -

Motor Circuits in Action: Specification, Connectivity, and Function
Neuron Review Motor Circuits in Action: Specification, Connectivity, and Function Silvia Arber1,2,* 1Biozentrum, Department of Cell Biology, University of Basel, Klingelbergstrasse 70, 4056 Basel, Switzerland 2Friedrich Miescher Institute for Biomedical Research, Maulbeerstrasse 66, 4058 Basel, Switzerland *Correspondence: [email protected] DOI 10.1016/j.neuron.2012.05.011 Mammalian motor behavior is enabled by a hierarchy of interleaved circuit modules constructed by interneu- rons in the spinal cord, sensory feedback loops, and bilateral communication with supraspinal centers. Neuronal subpopulations are specified through a process of precisely timed neurogenesis, acquisition of transcriptional programs, and migration to spatially confined domains. Developmental and genetic programs instruct stereotyped and highly specific connectivity patterns, binding functionally distinct neuronal subpop- ulations into motor circuit modules at all hierarchical levels. Recent work demonstrates that spatial organi- zation of motor circuits relates to precise connectivity patterns and that these patterns frequently correlate with specific behavioral functions of motor output. This Review highlights key examples of how develop- mental specification dictates organization of motor circuit connectivity and thereby controls movement. Introduction Through specific perturbations of functional or genetic differen- Movement is generated by the activity of neuronal circuits tiation programs in defined neuronal populations, recent studies collecting and integrating -

Neural Control of Movement: Motor Neuron Subtypes, Proprioception and Recurrent Inhibition
List of Papers This thesis is based on the following papers, which are referred to in the text by their Roman numerals. I Enjin A, Rabe N, Nakanishi ST, Vallstedt A, Gezelius H, Mem- ic F, Lind M, Hjalt T, Tourtellotte WG, Bruder C, Eichele G, Whelan PJ, Kullander K (2010) Identification of novel spinal cholinergic genetic subtypes disclose Chodl and Pitx2 as mark- ers for fast motor neurons and partition cells. J Comp Neurol 518:2284-2304. II Wootz H, Enjin A, Wallen-Mackenzie Å, Lindholm D, Kul- lander K (2010) Reduced VGLUT2 expression increases motor neuron viability in Sod1G93A mice. Neurobiol Dis 37:58-66 III Enjin A, Leao KE, Mikulovic S, Le Merre P, Tourtellotte WG, Kullander K. 5-ht1d marks gamma motor neurons and regulates development of sensorimotor connections Manuscript IV Enjin A, Leao KE, Eriksson A, Larhammar M, Gezelius H, Lamotte d’Incamps B, Nagaraja C, Kullander K. Development of spinal motor circuits in the absence of VIAAT-mediated Renshaw cell signaling Manuscript Reprints were made with permission from the respective publishers. Cover illustration Carousel by Sasha Svensson Contents Introduction.....................................................................................................9 Background...................................................................................................11 Neural control of movement.....................................................................11 The motor neuron.....................................................................................12 Organization -
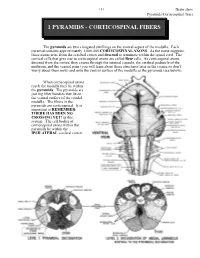
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Innovations Present in the Primate Interneuron Repertoire
Article Innovations present in the primate interneuron repertoire https://doi.org/10.1038/s41586-020-2781-z Fenna M. Krienen1,2 ✉, Melissa Goldman1,2, Qiangge Zhang2,3, Ricardo C. H. del Rosario2, Marta Florio1,2, Robert Machold4, Arpiar Saunders1,2, Kirsten Levandowski2,3, Heather Zaniewski2,3, Received: 19 July 2019 Benjamin Schuman4, Carolyn Wu3, Alyssa Lutservitz1,2, Christopher D. Mullally1,2, Nora Reed1,2, Accepted: 1 July 2020 Elizabeth Bien1,2, Laura Bortolin1,2, Marian Fernandez-Otero2,5, Jessica D. Lin2, Alec Wysoker2, James Nemesh2, David Kulp2, Monika Burns5, Victor Tkachev6,7,8, Richard Smith9,10, Published online: xx xx xxxx Christopher A. Walsh9,10, Jordane Dimidschstein2, Bernardo Rudy4,11, Leslie S. Kean6,7,8, Check for updates Sabina Berretta5,12,13, Gord Fishell2,14, Guoping Feng2,3 & Steven A. McCarroll1,2 ✉ Primates and rodents, which descended from a common ancestor around 90 million years ago1, exhibit profound diferences in behaviour and cognitive capacity; the cellular basis for these diferences is unknown. Here we use single-nucleus RNA sequencing to profle RNA expression in 188,776 individual interneurons across homologous brain regions from three primates (human, macaque and marmoset), a rodent (mouse) and a weasel (ferret). Homologous interneuron types—which were readily identifed by their RNA-expression patterns—varied in abundance and RNA expression among ferrets, mice and primates, but varied less among primates. Only a modest fraction of the genes identifed as ‘markers’ of specifc interneuron subtypes in any one species had this property in another species. In the primate neocortex, dozens of genes showed spatial expression gradients among interneurons of the same type, which suggests that regional variation in cortical contexts shapes the RNA expression patterns of adult neocortical interneurons. -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Glia Are a Unique Substrate for the in Vitro Growth of Central Nervous System Neurons1
0270.6474/84/0407-1892$02.00/O The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 4, No. 7, pp. 1892-1903 Printed in U.S.A. July 1984 GLIA ARE A UNIQUE SUBSTRATE FOR THE IN VITRO GROWTH OF CENTRAL NERVOUS SYSTEM NEURONS1 MARK NOBLE,*s2 JUIN FOK-SEANG,$ AND 31M COHENS * Department of Clinical Neurology, Institute of Neurology, London WClN 3BG, England and $ Medical Research Council Neuroimmunology Project, Department of Zoology, University College London, London WClE 6BT, England Received July 6,1983; Revised November 29, 1983; Accepted February 7, 1984 Abstract We have examined the consequences of surface interactions with glial and nonglial cells on the in vitro growth of CNS neurons. When cerebellar or spinal cord cells were plated onto monolayers highly enriched in cortical astrocytes or sciatic nerve Schwann cells, neurons generally grew as single cells and showed relatively little tendency to aggregate. Similarly, neurites showed little tendency to fasciculate. In contrast, when plated onto fibroblast, heart muscle-fibroblast, or astrocyte-free meningeal monolayers, neurons rapidly aggregated, and neurite outgrowth was primarily in large fascicles. There were no glia detectable in the majority of aggregates or fascicles, suggesting that aggregation and fasciculation were due to interactions between neurons. Neurite outgrowth over 24 hr was also greater on astrocytes than on nonglia. Whether or not aggregation and fasciculation occurred was due to surface properties of the glial and nonglial cells. When neurons were added to astrocyte and nonglial monolayers growing in medium conditioned by a large excess of co-cultured nonglia or astrocytes, respectively, the pattern of neuronal growth was determined by the type of monolayer with which the neurons were in contact. -

Relating Reflex Gain Modulation in Posture Control to Underlying Neural Network Properties Using a Neuromusculoskeletal Model
J Comput Neurosci (2011) 30:555–565 DOI 10.1007/s10827-010-0278-8 Relating reflex gain modulation in posture control to underlying neural network properties using a neuromusculoskeletal model Jasper Schuurmans · Frans C. T. van der Helm · Alfred C. Schouten Received: 25 March 2010 / Revised: 1 September 2010 / Accepted: 14 September 2010 / Published online: 24 September 2010 © The Author(s) 2010. This article is published with open access at Springerlink.com Abstract During posture control, reflexive feedback the neural, sensory and synaptic parameters on the joint allows humans to efficiently compensate for unpre- dynamics. The results showed that the lumped reflex dictable mechanical disturbances. Although reflexes gains positively correlate to their most direct neural are involuntary, humans can adapt their reflexive set- substrates: the velocity gain with Ia afferent velocity tings to the characteristics of the disturbances. Reflex feedback, the positional gain with muscle stretch over modulation is commonly studied by determining reflex II afferents and the force feedback gain with Ib afferent gains: a set of parameters that quantify the contribu- feedback. However, position feedback and force feed- tions of Ia, Ib and II afferents to mechanical joint be- back gains show strong interactions with other neural havior. Many mechanisms, like presynaptic inhibition and sensory properties. These results give important and fusimotor drive, can account for reflex gain mod- insights in the effects of neural properties on joint ulations. The goal of this study was to investigate the dynamics and in the identifiability of reflex gains in effects of underlying neural and sensory mechanisms experiments. on mechanical joint behavior. -

Synaptic Transmission Between Dorsal Root Ganglion and Dorsal
0270.6474/85/0508~2281$02.00/0 The Journal of Neuroscrence Copyright 0 Society for Neuroscrence Vol. 5, No 8, pp. 2281-2289 Printed rn U S.A. August 1985 Synaptic Transmission between Dorsal Root Ganglion and Dorsal Horn Neurons in Culture: Antagonism of Monosynaptic Excitatory Postsynaptic Potentials and Glutamate Excitation by Kynurenate’ C. E. JAHR AND T. M. JESSELL* Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115 Abstract Randic, 1984). Several lines of evidence suggest that the fast EPSPs in spinal cord neurons evoked by primary afferent stimulation are intracellular recording techniques have been used to pro- mediated by L-glutamate or by compounds with similar postsynaptic vide information on the identity of excitatory sensory trans- actions. lontophoretic and pressure applications of L-glutamate de- mitters released at synapses formed between dorsal root polarize the majority of mammalian spinal neurons in vivo and in ganglion (DRG) and dorsal horn neurons maintained in cell vitro (Ransom et al., 1977b; Watkins and Evans, 1981; Salt and Hill, culture. Explants of embryonic rat DRG were added to dis- 1983) with a reversal potential (Mayer and Westbrook, 1984) similar sociated cultures of embryonic dorsal horn neurons and to that of the EPSP evoked by dorsal root ganglion (DRG) neuron synaptic potentials were recorded intracellularly from dorsal stimulation (Engberg and Marshall, 1979; Finkel and Redman, 1983; horn neurons after DRG explant stimulation. More than 80% MacDonald et al., 1983). Biochemical analysis has demonstrated a of dorsal horn neurons within 1 mm of DRG explants received higher concentration of L-glutamate in dorsal than in ventral roots at least one fast, DRG-evoked, monosynaptic input. -
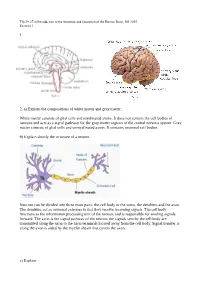
1. 2. A) Explain the Compositions of White Matter and Gray
Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 1. 2. a) Explain the compositions of white matter and gray matter. White matter consists of glial cells and myelinated axons. It does not contain the cell bodies of neurons and acts as a signal pathway for the gray matter regions of the central nervous system. Gray matter consists of glial cells and unmyelinated axons. It contains neuronal cell bodies. b) Explain shortly the structure of a neuron. Neurons can be divided into three main parts: the cell body or the soma, the dendrites and the axon. The dendrites act as neuronal antennas in that they receive incoming signals. The cell body functions as the information processing unit of the neuron, and is responsible for sending signals forward. The axon is the signal pathway of the neuron; the signals sent by the cell body are transmitted along the axon to the axon terminals located away from the cell body. Signal transfer is along the axon is aided by the myelin sheath that covers the axon. c) Explain: Tfy-99.2710 Introduction to the Structure and Operation of the Human Brain, fall 2015 Exercise 1 i) myelin Membrane that wraps around axons. Formed from glial support cells: oligodendrogolia in the central nervous system and Schwann cells in the peripheral nervous system. Myelin facilitates signal transfer along axons by allowing action potentials to skip between the nodes of Ranvier (saltatory conduction). ii) receptor Molecule specialized in receiving a chemical signal by binding a specific neurotransmitter. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.