Corticospinal Tract Location in Internal Capsule of Human Brain: Di¡Usion Tensor Tractography and Functional MRI Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -
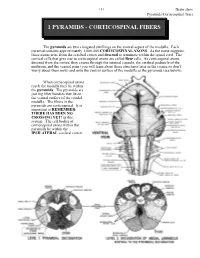
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

GLOSSARY Glossary Adapted with Permission from R
GLOSSARY Glossary adapted with permission from R. Kalb (ed.) Multiple Sclerosis: The Questions You Have: The Answers You Need (5th ed.) New York: Demos Medical Publishing, 2012. This glossary is available in its entirety (as well as additional MS terms) online at nationalMSsociety.org/glossary. 106 | KNOWLEDGE IS POWER 106 | KNOWLEDGE IS POWER Americans with Disabilities Act Blood-brain barrier (ADA) A semi-permeable cell layer around The first comprehensive legislation blood vessels in the brain and spinal to prohibit discrimination on the cord that prevents large molecules, basis of disability. The ADA (passed immune cells, and potentially in 1990) guarantees full participation damaging substances and disease- in society to people with disabilities. causing organisms (e.g., viruses) from The four areas of social activity passing out of the blood stream into the covered by the ADA are employment; central nervous system (brain, spinal public services and accommodations; cord and optic nerves). A break in the transportation; and communications blood-brain barrier may underlie the Autoimmune(e.g., telephone disease services). Centraldisease process nervous in system MS. A process in which the body’s immune The part of the nervous system that system causes illness by mistakenly includes the brain, optic nerves, and attacking healthy cells, organs or tissues Cerebrospinalspinal cord. fluid (CSF) in the body that are essential for good health. In multiple sclerosis, the specific antigen — or target — that the immune A watery, colorless, clear fluid that cells are sensitized to attack remains bathes and protects the brain and unknown, which is why MS is considered spinal cord. -

Progress and Challenges in Probing the Human Brain
University of Pennsylvania ScholarlyCommons Neuroethics Publications Center for Neuroscience & Society 10-2015 Progress and Challenges in Probing the Human Brain Russell A. Poldrack Martha J. Farah University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/neuroethics_pubs Part of the Bioethics and Medical Ethics Commons, Neuroscience and Neurobiology Commons, and the Neurosciences Commons Recommended Citation Poldrack, R. A., & Farah, M. J. (2015). Progress and Challenges in Probing the Human Brain. Nature, 526 (7573), 371-379. http://dx.doi.org/10.1038/nature15692 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/neuroethics_pubs/136 For more information, please contact [email protected]. Progress and Challenges in Probing the Human Brain Abstract Perhaps one of the greatest scientific challenges is to understand the human brain. Here we review current methods in human neuroscience, highlighting the ways that they have been used to study the neural bases of the human mind. We begin with a consideration of different levels of description relevant to human neuroscience, from molecules to large-scale networks, and then review the methods that probe these levels and the ability of these methods to test hypotheses about causal mechanisms. Functional MRI is considered in particular detail, as it has been responsible for much of the recent growth of human neuroscience research. We briefly er view its inferential strengths and weaknesses and present examples of new analytic approaches that allow inferences beyond simple localization of psychological processes. Finally, we review the prospects for real-world applications and new scientific challenges for human neuroscience. -

Basic Brain Anatomy
Chapter 2 Basic Brain Anatomy Where this icon appears, visit The Brain http://go.jblearning.com/ManascoCWS to view the corresponding video. The average weight of an adult human brain is about 3 pounds. That is about the weight of a single small To understand how a part of the brain is disordered by cantaloupe or six grapefruits. If a human brain was damage or disease, speech-language pathologists must placed on a tray, it would look like a pretty unim- first know a few facts about the anatomy of the brain pressive mass of gray lumpy tissue (Luria, 1973). In in general and how a normal and healthy brain func- fact, for most of history the brain was thought to be tions. Readers can use the anatomy presented here as an utterly useless piece of flesh housed in the skull. a reference, review, and jumping off point to under- The Egyptians believed that the heart was the seat standing the consequences of damage to the structures of human intelligence, and as such, the brain was discussed. This chapter begins with the big picture promptly removed during mummification. In his and works down into the specifics of brain anatomy. essay On Sleep and Sleeplessness, Aristotle argued that the brain is a complex cooling mechanism for our bodies that works primarily to help cool and The Central Nervous condense water vapors rising in our bodies (Aristo- tle, republished 2011). He also established a strong System argument in this same essay for why infants should not drink wine. The basis for this argument was that The nervous system is divided into two major sec- infants already have Central nervous tions: the central nervous system and the peripheral too much moisture system The brain and nervous system. -

Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: a Comparative in Situ Hybridization Analysis
The Journal of Neuroscience, March 1993. 73(3): 1258-1279 Gene Expression of Prohormone and Proprotein Convertases in the Rat CNS: A Comparative in situ Hybridization Analysis Martin K.-H. Schafer,i-a Robert Day,* William E. Cullinan,’ Michel Chri?tien,3 Nabil G. Seidah,* and Stanley J. Watson’ ‘Mental Health Research Institute, University of Michigan, Ann Arbor, Michigan 48109-0720 and J. A. DeSeve Laboratory of *Biochemical and 3Molecular Neuroendocrinology, Clinical Research Institute of Montreal, Montreal, Quebec, Canada H2W lR7 Posttranslational processing of proproteins and prohor- The participation of neuropeptides in the modulation of a va- mones is an essential step in the formation of bioactive riety of CNS functions is well established. Many neuropeptides peptides, which is of particular importance in the nervous are synthesized as inactive precursor proteins, which undergo system. Following a long search for the enzymes responsible an enzymatic cascade of posttranslational processing and mod- for protein precursor cleavage, a family of Kexin/subtilisin- ification events during their intracellular transport before the like convertases known as PCl, PC2, and furin have recently final bioactive products are secreted and act at either pre- or been characterized in mammalian species. Their presence postsynaptic receptors. Initial endoproteolytic cleavage occurs in endocrine and neuroendocrine tissues has been dem- C-terminal to pairs of basic amino acids such as lysine-arginine onstrated. This study examines the mRNA distribution of (Docherty and Steiner, 1982) and is followed by the removal these convertases in the rat CNS and compares their ex- of the basic residues by exopeptidases. Further modifications pression with the previously characterized processing en- can occur in the form of N-terminal acetylation or C-terminal zymes carboxypeptidase E (CPE) and peptidylglycine a-am- amidation, which is essential for the bioactivity of many neu- idating monooxygenase (PAM) using in situ hybridization ropeptides. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain. -

Neuroscience
NEUROSCIENCE SCIENCE OF THE BRAIN AN INTRODUCTION FOR YOUNG STUDENTS British Neuroscience Association European Dana Alliance for the Brain Neuroscience: the Science of the Brain 1 The Nervous System P2 2 Neurons and the Action Potential P4 3 Chemical Messengers P7 4 Drugs and the Brain P9 5 Touch and Pain P11 6 Vision P14 Inside our heads, weighing about 1.5 kg, is an astonishing living organ consisting of 7 Movement P19 billions of tiny cells. It enables us to sense the world around us, to think and to talk. The human brain is the most complex organ of the body, and arguably the most 8 The Developing P22 complex thing on earth. This booklet is an introduction for young students. Nervous System In this booklet, we describe what we know about how the brain works and how much 9 Dyslexia P25 there still is to learn. Its study involves scientists and medical doctors from many disciplines, ranging from molecular biology through to experimental psychology, as well as the disciplines of anatomy, physiology and pharmacology. Their shared 10 Plasticity P27 interest has led to a new discipline called neuroscience - the science of the brain. 11 Learning and Memory P30 The brain described in our booklet can do a lot but not everything. It has nerve cells - its building blocks - and these are connected together in networks. These 12 Stress P35 networks are in a constant state of electrical and chemical activity. The brain we describe can see and feel. It can sense pain and its chemical tricks help control the uncomfortable effects of pain. -

Brain Comparisons Teacher's Guide
Teacher’s Guide Written by Leslie Miller, Ph.D., Barbara Tharp, M.S., Judith Dresden, M.S., Katherine Taber, Ph.D., Karen Kabnick, Ph.D., and Nancy Moreno, Ph.D. © 2015 Baylor College of Medicine TEACHER’S GUIDE Written by Leslie Miller, Ph.D., Barbara Tharp, M.S., Judith Dresden, M.S. Katherine Taber, Ph.D., Karen Kabnick, Ph.D., and Nancy Moreno, Ph.D. BioEd Teacher Resources from the Center for Educational Outreach Baylor College of Medicine ISBN: 978-1-888997-98-9 © 2015 Baylor College of Medicine © 2015 Baylor College of Medicine. All rights reserved. Fifth edition. First edition published 1992. Printed in the United States of America. ISBN: 978-1-888997-98-9 Teacher Resources from the Center for Educational Outreach at Baylor College of Medicine. The mark “BioEd” is a service mark of Baylor College of Medicine. The marks “BrainLink” and “NeuroExplorers” are registered trademarks of Baylor College of Medicine. No part of this book may be reproduced by any mechanical, photographic or electronic process, or in the form of an audio recording, nor may it be stored in a retrieval system, transmitted or otherwise copied for public or private use without prior written permission of the publisher, except for classroom use. The activities described in this book are intended for school-age children under direct supervision of adults. The authors, Baylor College of Medicine and the publisher cannot be responsible for any accidents or injuries that may result from conduct of the activities, from not specifically following directions, or from ignoring cautions contained in the text. -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

Two Fiber Pathways Connecting Amygdala and Prefrontal Cortex in Humans and Monkeys
bioRxiv preprint doi: https://doi.org/10.1101/561811; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Two fiber pathways connecting amygdala and prefrontal cortex in humans and monkeys Davide Folloni1,2*, Jérôme Sallet1,2, Alexandre A. Khrapitchev3, Nicola R. Sibson3, Lennart Verhagen1,2†, Rogier B. Mars2,4† 1Wellcome Integrative Neuroimaging (WIN), Department of Experimental Psychology, University of Oxford, Oxford, United Kingdom 2Wellcome Integrative Neuroimaging (WIN), Centre for Functional MRI of the Brain (FMRIB), Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom 3Cancer Research UK and Medical Research Council Oxford Institute for Radiation Oncology, Department of Oncology, University of Oxford, Oxford, United Kingdom 4Donders Institute for Brain, Cognition and Behaviour, Radboud University Nijmegen, Nijmegen, The Netherlands †Authors contributed equally to the work *To whom correspondence should be addressed: Address: Davide Folloni, Department of Experimental Psychology, University of Oxford, Tinsley Building, Mansfield Road, Oxford, OX1 3SR, UK E-mail: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/561811; this version posted March 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract The interactions between amygdala and prefrontal cortex are pivotal to many neural processes involved in learning, decision-making, emotion, and social regulation. -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or