Degeneration of Axons in the Corticospinal Tract Secondary To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -
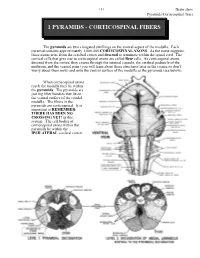
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Visualization of the Pyramidal Decussation Utilizing Diffusion
OPEN ACCESS RESEARCH Visualization of the pyramidal decussation utilizing diffusion tensor imaging: a feasibility study utilizing generalized q-sampling imaging Erik H Middlebrooks1,*, Jeffrey A Bennett1, Sharatchandra Bidari1, and Alissa Old Crow1 1Department of Radiology, University of Florida College of Medicine, Gainesville, Florida, USA *corresponding author ([email protected]) Abstract Background: The delineating point of the end of the brainstem and beginning of the spinal cord is known as the cervicomedullary junction (CMJ). This point is defined by the decussation of the pyramidal tracts. Abnormal configuration and location of the CMJ have both been implicated in disease processes such as Chiari malformation. Unfortunately, the CMJ is not directly visualized on contemporary imaging techniques. Diffusion tensor imaging (DTI) has given us the ability to directly visualize white matter tracts, but suffers from difficulties with visualizing crossing fibers. Many advanced techniques for visualizing crossing fibers utilize substantially long imaging times or non-clinical magnet strengths making clinical applicability limited. Objective: This study inves- tigates the use of generalized q-sampling imaging with diffusion decomposition on standard DTI acquisitions at 3 Tesla to demonstrate the pyramidal decussation. Methods: Three differing DTI protocols were analyzed in vivo with scan times of 17 minutes, 10 minutes, and 5.5 minutes. Data was processed with standard DTI post-processing, as well as generalized q-sampling imaging with diffusion decomposition. Results: The results of the study show that the pyramidal decussation can be reliably visualized using generalized q-sampling imaging and diffusion decomposition with scan times as low at 10 minutes. Conclusion: Utilizing generalized q-sampling post-processing, the pyramidal decussation can be reliably visualized using clinically feasible DTI sequences with scan times as low as 10 minutes. -

Major Motor & Sensory Projections
Major Motor & Sensory Projections Neuroanatomy > Brainstem > Brainstem MAJOR MOTOR & SENSORY PROJECTIONS  FULL TEXT OVERVIEW • Here, we will learn a consolidated view of the major descending (motor) and ascending (sensory) pathways from the cerebrum through the brainstem into the spinal cord. • Start a table, specify that we will learn about the following major pathway projections: Motor • Corticospinal tract (CST) • Corticobulbar (aka corticonuclear) tract Sensory • Posterior column/medial lemniscus • Anterolateral system (spinothalamic) tract • Trigeminothalamic tract MOTOR PATHWAYS Anatomical Structures Begin with the motor pathways. • Label the superior/inferior axes. • Draw a coronal view of the right brain. • Then, the brainstem. • The upper cervical spinal cord. 1 / 5 • And the lumbar cord. Innervation • Start another table. Write that for the motor fibers: • The corticospinal tract fibers innervate the body via spinal motor neurons. - Specify that the lateral CST innervates distal musculature for fine motor movements - Whereas the anterior CST innervates proximal musculature for gross motor movements. • The corticobulbar fibers (aka corticonuclear fibers) innervate the face via the CNs. Projections • Now, demarcate the internal capsule deep within the cerebrum – the motor fibers consolidate here before entering the ipsilateral cerebral peduncle in the midbrain. • Next, draw the twisting descent of each fiber group through the subcortical white matter. • Show that the facial fibers [RED] emerge from the lateral convexity, descend medially and, generally, decussate to synapse on different cranial nerve nuclei throughout their descent. • Then, show that the leg fibers [GREEN] emerge paracentrally, descend laterally through the brainstem into the ipsilateral medullary pyramid. • Show that the arm fibers [BLUE] emerge from the upper convexity, descend in between the facial and leg fibers, medial to the leg fibers through the brainstem into the ipsilateral medullary pyramid. -

Reaction to Injury & Regeneration
Reaction to Injury & Regeneration Steven McLoon Department of Neuroscience University of Minnesota 1 The adult mammalian central nervous system has the lowest regenerative capacity of all organ systems. 2 3 Reaction to Axotomy Function distal to the axon cut is lost. (immediate) 4 Reaction to Axotomy • Spinal cord injury results in an immediate loss of sensation and muscle paralysis below the level of the injury. Spinal cord injury can be partial or complete, and the sensory/motor loss depends on which axons are injured. • Peripheral nerve injury results in an immediate loss of sensation and muscle paralysis in the areas served by the injured nerve distal to the site of injury. 5 Reaction to Axotomy K+ leaks out of the cell and Na+/Ca++ leak into the cell. (seconds) Proximal and distal segments of the axon reseal slightly away from the cut ends. (~2 hrs) Subsequent anterograde & retrograde effects … 6 Anterograde Effects (Wallerian Degeneration) Axon swells. (within 12 hrs) Axolema and mitochondria begin to fragment. (within 3 days) Myelin not associated with a viable axon begins to fragment. (within 1 wk) Astrocytes or Schwann cells proliferate (within 1 wk), which can continue for over a month. Results in >10x the original number of cells. Microglia (or macrophages in the PNS) invade the area. Glia and microglia phagocytize debris. (1 month in PNS; >3 months in CNS) 7 Anterograde Effects (Wallerian Degeneration) Degradation of the axon involves self proteolysis: In the ‘Wallerian degeneration slow’ (Wlds) mouse mutation, the distal portion of severed axons are slow to degenerate; the dominant mutation involves a ubiquitin regulatory enzyme. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body. -

Testing Upper Motor Neuron Function in Amyotrophic Lateral Sclerosis: the Most Difficult Task of Neurophysiology
Scientific Commentaries Brain 2012: 135; 2579–2584 | 2581 Testing upper motor neuron function in amyotrophic lateral sclerosis: the most difficult task of neurophysiology Clinical signs of upper motor neuron involvement are an essential contrast is potentially very effective for exploring neuronal inter- observation to support the diagnosis of amyotrophic lateral scler- connection dysfunction in amyotrophic lateral sclerosis, but still osis. However, clinical signs of upper motor neuron can be difficult needs more investigation; and novel neuroinflammatory and in- to elicit in patients with motor neuron disease. One postulated hibitory positron emission tomography ligands might have utility reason for this problem is the presence of marked limb weakness in the future (Turner, 2012). However, expense and practical Downloaded from https://academic.oup.com/brain/article/135/9/2581/331426 by guest on 23 September 2021 and amyotrophy in motor neuron disease. This has been observed issues limit the use of these sophisticated imaging techniques to in patients with genetic mutations and clear-cut pathological evi- a few highly specialized centres. Thus far, therefore, no method to dence of upper and lower motor neuron degeneration. Less com- investigate upper motor neuron function has proved useful and monly, it has been recognized that the pattern of upper motor applicable as a measure of efficacy in clinical trials, despite some neuron lesion in amyotrophic lateral sclerosis is rather different enthusiasm for the threshold tracking transcranial magnetic stimu- from other conditions, in which there is damage to other descend- lation as a marker of early diagnosis. ing motor fibres from extra-Rolandic motor cortical areas (Swash, EMG is also not the preferred method for assessing upper motor 2012). -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or -

Functional Significance of the Ventral and Lateral Funiculi in the Frog's Spinal Cord
ACTA NEUROBIOL. EXP. 1976, 36: 593-812 FUNCTIONAL SIGNIFICANCE OF THE VENTRAL AND LATERAL FUNICULI IN THE FROG'S SPINAL CORD Zofia AFELT Department of Neurophysiology, Nencki Institute of Experimental Biology Warsaw, Poland Abstract. The spinal cord was transected at the level of calamus scriptorius either completely (spinal preparation) or partially (funicular preparation). In the operated frogs and in normal controls cranial and peripheral receptive fields were stimulated by natural stimuli. Results indicated that the information necessary for somatic activation of the protective flight ascends in the dorsolateral funiculus, which can be characterized as an ascending part of the reflex arc of somatotopically guided avoidance behavior. The tectal region is apparently a center of responses. The information necessary for activating different patterns of locomotion adequate to environmental conditions goes unilaterally through the ventral and ventrolateral funiculus. A diminished specifi- city of these motor patterns observed after injury of the ventrolateral funiculus seems to be related to a reduced feedback from the periphery. Lacking of aquatic pattern of limb movements (even when terrestrial patterns of locomotion were undisturbed), and drowning in water seem to be related to an injury of a vestibulo-spinal system in the ventral funiculus. It is concluded that the ventrolateral area of the frog spinal cord, i.e., the area between median fissure and dorsal root, forms a clo- sed system providing afferent and efferent information sufficient to evoke normal behavior. INTRODUCTION A high autonomy of the spinal cord function in lower vertebrates has been repeatedly stressed since the last century. However, even in the most primitive forms, a supraspinal control over body movement has 594 Z.