Visualization of the Pyramidal Decussation Utilizing Diffusion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Corticospinal Fibers
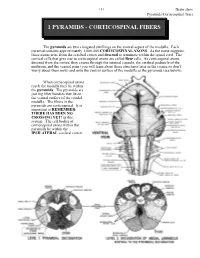
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or -

Amyotrophic Lateral Sclerosis (ALS)
Amyotrophic Lateral Sclerosis (ALS) There are multiple motor neuron diseases. Each has its own defining features and many characteristics that are shared by all of them: Degenerative disease of the nervous system Progressive despite treatments and therapies Begins quietly after a period of normal nervous system function ALS is the most common motor neuron disease. One of its defining features is that it is a motor neuron disease that affects both upper and lower motor neurons. Anatomical Involvement ALS is a disease that causes muscle atrophy in the muscles of the extremities, trunk, mouth and face. In some instances mood and memory function are also affected. The disease operates by attacking the motor neurons located in the central nervous system which direct voluntary muscle function. The impulses that control the muscle function originate with the upper motor neurons in the brain and continue along efferent (descending) CNS pathways through the brainstem into the spinal cord. The disease does not affect the sensory or autonomic system because ALS affects only the motor systems. ALS is a disease of both upper and lower motor neurons and is diagnosed in part through the use of NCS/EMG which evaluates lower motor neuron function. All motor neurons are upper motor neurons so long as they are encased in the brain or spinal cord. Once the neuron exits the spinal cord, it operates as a lower motor neuron. 1 Upper Motor Neurons The upper motor neurons are derived from corticospinal and corticobulbar fibers that originate in the brain’s primary motor cortex. They are responsible for carrying impulses for voluntary motor activity from the cerebral cortex to the lower motor neurons. -

Quantifying Nerve Decussation Abnormalities in the Optic Chiasm T Robert J
NeuroImage: Clinical 24 (2019) 102055 Contents lists available at ScienceDirect NeuroImage: Clinical journal homepage: www.elsevier.com/locate/ynicl Quantifying nerve decussation abnormalities in the optic chiasm T Robert J. Puzniaka, Khazar Ahmadia, Jörn Kaufmannb, Andre Gouwsc, Antony B. Morlandc,d, ⁎ Franco Pestillie,1, Michael B. Hoffmanna,f,1, a Department of Ophthalmology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany b Department of Neurology, Otto-von-Guericke-University Magdeburg, Magdeburg, Germany c York Neuroimaging Centre, Department of Psychology, University of York, York, United Kingdom d York Biomedical Research Institute, University of York, York, United Kingdom e Department of Psychological and Brain Sciences, Program in Neuroscience and Program in Cognitive Science, Indiana University, Bloomington, USA f Center for Behavioral Brain Sciences, Magdeburg, Germany ARTICLE INFO ABSTRACT Keywords: Objective: The human optic chiasm comprises partially crossing optic nerve fibers. Here we used diffusion MRI Optic chiasm (dMRI) for the in-vivo identification of the abnormally high proportion of crossing fibers found intheoptic Albinism chiasm of people with albinism. Diffusion MRI Methods: In 9 individuals with albinism and 8 controls high-resolution 3T dMRI data was acquired and analyzed Functional MRI with a set of methods for signal modeling [Diffusion Tensor (DT) and Constrained Spherical Deconvolution Crossing nerves (CSD)], tractography, and streamline filtering (LiFE, COMMIT, and SIFT2). The number of crossing andnon- crossing streamlines and their weights after filtering entered ROC-analyses to compare the discriminative power of the methods based on the area under the curve (AUC). The dMRI results were cross-validated with fMRI estimates of misrouting in a subset of 6 albinotic individuals. -

Mapping the Corticoreticular Pathway from Cortex-Wide Anterograde Axonal
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.23.449661; this version posted June 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Mapping the corticoreticular pathway from cortex-wide anterograde axonal tracing in the mouse Pierce Boyne, PT, DPT, PhD, NCS;1 Oluwole O. Awosika, MD, MS;2 Yu Luo, PhD3 1Department of Rehabilitation, Exercise and Nutrition Sciences, College of Allied Health Sciences, University of Cincinnati, Cincinnati, OH, 45267, USA 2Department of Neurology and Rehabilitation Medicine, College of Medicine, University of Cincinnati, Cincinnati, OH, 45267, USA 3Department of Molecular Genetics, Biochemistry and Microbiology, College of Medicine, University of Cincinnati, Cincinnati, OH, 45267, USA Key words: motor activity, locomotion, postural balance, pyramidal tracts, extrapyramidal tracts, brain mapping Grant support: PB is supported by NIH grant R01HD093694. OOA is supported by an American Academy of Neurology Career Development Award. YL is supported by NIH grant R01NS107365. Corresponding author: Pierce Boyne, PT, DPT, PhD, NCS Health Sciences Building, room 267 3225 Eden Ave. Cincinnati, OH, 45267-0394 [email protected] 513-558-7499 (phone); 513-558-7474 (fax) 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.06.23.449661; this version posted June 23, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 ABSTRACT 2 The corticoreticular pathway (CRP) has been implicated as an important mediator of 3 motor recovery and rehabilitation after central nervous system damage. -

Rubrospinal Tract
LECTURE IV: Physiology of Motor Tracts EDITING FILE GSLIDES IMPORTANT MALE SLIDES EXTRA FEMALE SLIDES LECTURER’S NOTES 1 PHYSIOLOGY OF MOTOR TRACTS Lecture Four In order to initiate any type of voluntary movement there will be 2 levels of neuron that your body will use and they are: Upper Motor Neurons (UMN) Lower Motor Neurons (LMN) These are the motor These are the motor neurons whose cell bodies neurons of the spinal lie in the motor cortex, or cord (AHCs) and brain brainstem, and they stem motor nuclei of the activate the lower motor cranial nerves that neuron innervates skeletal muscle directly. Figure 4-1 The descending motor system (pyramidal,Extrapyramidal )has a number of important sets these are named according to the origin of their cell bodies and their final destination; Originates from the cerebral ● The rest of the descending motor pathways 1 cortex and descends to the pyramidal do not travel through the medullary pyramids spinal cord (the corticospinal extra and are therefore collectively gathered under tract) passes through the the heading:“the extrapyramidal tracts” pyramids of the medulla and ● Responsible for subconscious gross therefore has been called the “the pyramidal movements(swinging of arms during walking) pyramidal tract ” DESCENDING MOTOR SYSTEM PYRAMIDAL EXTRAPYRAMIDAL Corticospinal Corticobulbar Rubrospinal Vestibulospinal Tectospinal tracts tracts tracts tracts tracts Reticulospinal Olivospinal tract Tract FOOTNOTES 1. They are collections of white matter in the medulla that appear triangular due to crossing of motor tracts. Therefore they are termed “medullary pyramids”. 2 PHYSIOLOGY OF MOTOR TRACTS Lecture Four MOTOR AREAS Occupies the Precentral Area of representation Gyrus & contains large, is proportional with the giant highly excitable complexity of function Betz cells. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

MITOCW | MIT9 14S09 Lec16-Mp3
MITOCW | MIT9_14S09_lec16-mp3 The following content is provided under a Creative Commons license. Your support will help MIT OpenCourseWare continue to offer high quality educational resources for free. To make a donation, or view additional materials from hundreds of MIT courses, visit MIT OpenCourseWare at ocw.mit.edu. PROFESSOR: OK, today let's continue with the motor system. The quiz for this week will be posted. Just a few questions. One of them might require you to do a little bit on the web, but it should be pretty easy for you to finish it by-- I'll accept them through Saturday. We were talking about hierarchical control of local motor behavior. And I pointed out how local motor pattern controllers and initiators in the hypothalamus or sub- thalamus-- they call it the mid-brain-- get a lot of input from the end-brain, as I show here in blue. And I just want to point out that this picture might lead you to believe that locomotion is always originating in the end-brain. Show it being initiated by olfactory inputs, other kinds of sensory inputs that come in through the older pathways through the old thalamus and striatum. And the newer pathways reaching the neocortex. But I note here that some inputs from the visual system, they actually reach that area, the mid-brain locomotor area more directly without going through the striatum. They come through a part of the [? pretectural ?] [? legion ?]. And it's very likely that other systems do the same, but we know-- remember those lesion experiments I talked about some time ago? Animals with decerebrations where the whole end-brain was removed? They didn't initiate much locomotion at all. -

Developmental Determinants at the Mammalian Optic Chiasm
- Feature Article Developmental Determinants at the Mammalian Optic Chiasm Ft. W. Guillery,’ C. A. Mason,* and J. S. H. Taylor’ ‘Department of Human Anatomy, University of Oxford, Oxford OX1 3QX, United Kingdom and *Departments of Pathology, Anatomy, and Cell Biology, Centre for Neurobiology and Behaviour, College of Physicians and Surgeons, Columbia University, New York 10032 The optic chiasm is the point at the ventral midline of the di- ber of mutants are known that show well-defined abnormalities encephalon where the nerve fibers from the two eyes meet. Clas- of the optic chiasm. The chiasm can no longer be regarded sim- sically, it has been known as the place where groups of retinal ply as the region where some axons cross their partners from axons segregate to pass into the optic tract on either the same the other eye, and others diverge into an uncrossed course. There or the opposite side of the brain (Figs. 1, 2). This segregation is is now an opportunity to look more closely at the cellular and dependent upon the retinal position of the ganglion cells from molecular events producing the characteristic and rigidly cho- which the axons arise: axons from the nasal retina all cross to reographed patterns of axonal growth. The development of the the opposite side, whereas many from the temporal or ventro- optic chiasm and the reorganization of retinotopic order that oc- temporal part of the retina (temporal crescent) remain uncrossed. curs within region of the chiasm can now be seen in terms of a This segregation of the axons into a crossed and uncrossed com- number of discrete steps, each probably relatively simple in it- ponent allows the appropriate bilateral connections that underlie self, which together produce the final developmental sequence. -

Decussation Geometries in the Goldfish Nervous System
Proc. Natl. Acad. Sci. USA Vol. 76, No. 8, pp. 4131-4135, August 1979 Neurobiology Decussation geometries in the goldfish nervous system: Correlation with probability of survival (brain asymmetries/lateralization of function/Mauthner's neuron/optic chiasm/natural selection) RICHARD L. ROTH Department of Biological Sciences, Stanford University, Stanford, California 94305 Communicated by Donald Kennedy, May 9, 1979 ABSTRACT In the goldfish, the optic nerve decussation assumed to be representative of goldfish available in the United occurs without intermingling of fibers from the two eyes. In States. The embryonic and larval specimens examined were two-thirds of juvenile and adult specimens, the left optic nerve derived from two lots of fertile eggs and, by the breeders' es- is dorsal at the midline. In about 60% of the specimens, the decussation of Mauthner's neuron also has a left-dorsal-to-right timates, represent the progeny of 9-14 spawning females.* (L/R) configuration. Concordance for decussation geometry is Despite this limited sampling of embryos and larvae, the results greater than 80%, with smaller specimens accounting for a presented below indicate that they, too, provide an adequate disproportionate number of discordant cases. In embryos and basis for generalization. very young larvae, the L/R configuration occurs in slightly less For minimization of sampling bias, most of the Spanish moss than 50% of o tic chiasmata and in slightly more than 50% of into each Mauthner's cell chiasmata, and there is no significant tendency containing the eggs was teased and cut portions, toward concordance. However, larval specimens that survive bearing 50-75 eggs.