Auditory and Vestibular Systems Objective • to Learn the Functional
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fibroblast Growth Factor (Fgf) Implication in the Neonatal Development of the Cochlear Innervation G
FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION G. Després, I. Jalenques, R. Romand To cite this version: G. Després, I. Jalenques, R. Romand. FIBROBLAST GROWTH FACTOR (FGF) IMPLICATION IN THE NEONATAL DEVELOPMENT OF THE COCHLEAR INNERVATION. Journal de Physique IV Proceedings, EDP Sciences, 1992, 02 (C1), pp.C1-173-C1-176. 10.1051/jp4:1992134. jpa-00251205 HAL Id: jpa-00251205 https://hal.archives-ouvertes.fr/jpa-00251205 Submitted on 1 Jan 1992 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. JOURNAL DE PHYSIQUE IV Colloque C1, suppICment au Journal de Physique 111, Volume 2, avril 1992 FIBROBLAST GROWMI FACTOR (FGF) IMPLICATION IN TIlE NEONATAL DEVELOPMENT OF THE COCIiLEAR INNERVATION G. DESPR~S,I. JALENQUES and R. ROMAND Laboratoire de Neurobiolc@e et Physwlogie du Dkeloppement, Universite'BIaise Pascal, F-63177Aubi2re ceder, France The presence of fibroblast growth factor-like protein has been investigated on cryostat sections from Sprague Dawley rat cochleae and auditory brainstem nuclei of various neonatal stages by indirect immunofluorescence and immunoperoxydase techniques with an antibody directed against the 1-24 amino-acid sequence of brain derived basic FGF. -
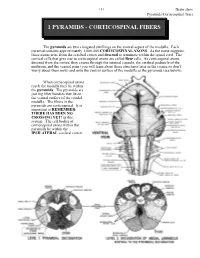
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Projections from the Trigeminal Nuclear Complex to the Cochlear Nuclei: a Retrograde and Anterograde Tracing Study in the Guinea Pig
Journal of Neuroscience Research 78:901–907 (2004) Projections From the Trigeminal Nuclear Complex to the Cochlear Nuclei: a Retrograde and Anterograde Tracing Study in the Guinea Pig Jianxun Zhou and Susan Shore* Department of Otolaryngology and Kresge Hearing Research Institute, University of Michigan, Ann Arbor, Michigan In addition to input from auditory centers, the cochlear cuneate nucleus innervation of cochlear nucleus has been nucleus (CN) receives inputs from nonauditory centers, hypothesized to convey information about head and pinna including the trigeminal sensory complex. The detailed position for the purpose of localizing a sound source in anatomy, however, and the functional implications of the space (Young et al., 1995). In addition, interactions be- nonauditory innervation of the auditory system are not tween somatosensory and auditory systems have been fully understood. We demonstrated previously that the linked increasingly to phantom sound perception, also trigeminal ganglion projects to CN, with terminal labeling known as tinnitus. This is demonstrated in the observa- most dense in the marginal cell area and secondarily in tions that injuries of the head and neck region can lead to the magnocellular area of the ventral cochlear nucleus the onset of tinnitus in patients with no hearing loss (VCN). We continue this line of study by investigating the (Lockwood et al., 1998). projection from the spinal trigeminal nucleus to CN in We demonstrated previously projections from the guinea pig. After injections of the retrograde tracers Flu- trigeminal ganglion to CN in guinea pigs (Shore et al., oroGold or biotinylated dextran amine (BDA) in VCN, 2000). Terminal labeling of trigeminal ganglion projec- labeled cells were found in the spinal trigeminal nuclei, tions to the CN was found to be most dense in the most densely in the pars interpolaris and pars caudalis marginal cell area and secondarily in the magnocellular with ipsilateral dominance. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -

5.1. Structure of the Spiral Ganglion
CHAPTER 5. INNERVATION OF THE ORGAN OF CORTI The investigation of nerve components of the acoustic system’s periph- eral part is so difficult methodologically that a whole series of questions which were solved long ago during the investigation of the other sensory system have not yet been clarified. The basic difficulty for the morphologists lies in the fact that the organ of Corti, together with its nerve elements, is located within the osseous tissue. In addition, it is in the shape of a spirally involut- ed geometrical figure. These structural peculiarities create considerable dif- ficulties during the determination of the connections between the types of peripheral and central neuron’s processes and bodies of the spiral ganglion of the cochlea. Therefore, most of the work on the cochlea’s innervation and the computation of the different element’s quantity demands an application of special methods, including graphical reconstruction of the serial sections. The Golgi method was and still remains the basic histological method of the organ of Corti’s innervation study, which has been supplemented by the cochlea’s electron-microscope investigations in the normal conditions and during the experimentally induced degenerations. 5.1. Structure of the spiral ganglion The neurons which innervate the auditory receptor cells form a spiral ganglion: a nerve-knot of the VIII pair’s acoustic part of the craniocerebral nerves. The ganglion fills the Rosental’s canal in the cochlea’s axis and re- peats the number of its spiral turns. The ganglionic neuron has, as a rule, a widened body with two processes: peripheral and central (Diagram 7). -

Multisensory Integration in the Dorsal Cochlear Nucleus: Unit Responses to Acoustic and Trigeminal Ganglion Stimulation
European Journal of Neuroscience, Vol. 21, pp. 3334–3348, 2005 ª Federation of European Neuroscience Societies Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation S. E. Shore Kresge Hearing Research Institute and Department of Otolaryngology, University of Michigan, 1301 East Ann Street, Ann Arbor, MI 48109, USA Keywords: auditory, guinea pig, multisensory, neural pathways, somatosensory, trigeminal Abstract A necessary requirement for multisensory integration is the convergence of pathways from different senses. The dorsal cochlear nucleus (DCN) receives auditory input directly via the VIIIth nerve and somatosensory input indirectly from the Vth nerve via granule cells. Multisensory integration may occur in DCN cells that receive both trigeminal and auditory nerve input, such as the fusiform cell. We investigated trigeminal system influences on guinea pig DCN cells by stimulating the trigeminal ganglion while recording spontaneous and sound-driven activity from DCN neurons. A bipolar stimulating electrode was placed into the trigeminal ganglion of anesthetized guinea pigs using stereotaxic co-ordinates. Electrical stimuli were applied as bipolar pulses (100 ls per phase) with amplitudes ranging from 10 to 100 lA. Responses from DCN units were obtained using a 16-channel, four-shank electrode. Current pulses were presented alone or preceding 100- or 200-ms broadband noise (BBN) bursts. Thirty percent of DCN units showed either excitatory, inhibitory or excitatory–inhibitory responses to trigeminal ganglion stimulation. When paired with BBN stimulation, trigeminal stimulation suppressed or facilitated the firing rate in response to BBN in 78% of units, reflecting multisensory integration. Pulses preceding the acoustic stimuli by as much as 95 ms were able to alter responses to BBN. -

Neuronal Organization in the Inferior Colliculus Revisited with Cell-Type- Dependent Monosynaptic Tracing
This Accepted Manuscript has not been copyedited and formatted. The final version may differ from this version. Research Articles: Systems/Circuits Neuronal organization in the inferior colliculus revisited with cell-type- dependent monosynaptic tracing Chenggang Chen1, Mingxiu Cheng1,2, Tetsufumi Ito3 and Sen Song1 1Tsinghua Laboratory of Brain and Intelligence (THBI) and Department of Biomedical Engineering, Beijing Innovation Center for Future Chip, Center for Brain-Inspired Computing Research, McGovern Institute for Brain Research, Tsinghua University, Beijing, 100084, China 2National Institute of Biological Sciences, Beijing, 102206, China 3Anatomy II, School of Medicine, Kanazawa Medical University, Uchinada, Ishikawa, 920-0293, Japan DOI: 10.1523/JNEUROSCI.2173-17.2018 Received: 31 July 2017 Revised: 2 February 2018 Accepted: 7 February 2018 Published: 24 February 2018 Author contributions: C.C., T.I., and S.S. designed research; C.C. and M.C. performed research; C.C. and T.I. analyzed data; C.C. wrote the first draft of the paper; C.C., T.I., and S.S. edited the paper; C.C., T.I., and S.S. wrote the paper. Conflict of Interest: The authors declare no competing financial interests. This work was supported by funding from the National Natural Science Foundation of China (31571095, 91332122, for S.S.), Special Fund of Suzhou-Tsinghua Innovation Leading Action (for S.S.), Beijing Program on the Study of Brain-Inspired Computing System and Related Core Technologies (for S.S.), Beijing Innovation Center for Future Chip (for S.S.), and Chinese Academy of Sciences Institute of Psychology Key Laboratory of Mental Health Open Research Grant (KLMH2012K02, for S.S.), grants from Ministry of Education, Science, and Culture of Japan (KAKENHI grant, Grant numbers 16K07026 and 16H01501; for T.I.), and Takahashi Industrial and Economic Research Foundation (for T.I.). -

Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat
The Journal of Neuroscience, December 15, 2002, 22(24):10891–10897 Direct Projections from Cochlear Nuclear Complex to Auditory Thalamus in the Rat Manuel S. Malmierca,1 Miguel A. Mercha´n,1 Craig K. Henkel,2 and Douglas L. Oliver3 1Laboratory for the Neurobiology of Hearing, Institute for Neuroscience of Castilla y Leo´ n and Faculty of Medicine, University of Salamanca, 37007 Salamanca, Spain, 2Wake Forest University School of Medicine, Department of Neurobiology and Anatomy, Winston-Salem, North Carolina 27157-1010, and 3University of Connecticut Health Center, Department of Neuroscience, Farmington, Connecticut 06030-3401 It is known that the dorsal cochlear nucleus and medial genic- inferior colliculus and are widely distributed within the medial ulate body in the auditory system receive significant inputs from division of the medial geniculate, suggesting that the projection somatosensory and visual–motor sources, but the purpose of is not topographic. As a nonlemniscal auditory pathway that such inputs is not totally understood. Moreover, a direct con- parallels the conventional auditory lemniscal pathway, its func- nection of these structures has not been demonstrated, be- tions may be distinct from the perception of sound. Because cause it is generally accepted that the inferior colliculus is an this pathway links the parts of the auditory system with prom- obligatory relay for all ascending input. In the present study, we inent nonauditory, multimodal inputs, it may form a neural have used auditory neurophysiology, double labeling with an- network through which nonauditory sensory and visual–motor terograde tracers, and retrograde tracers to investigate the systems may modulate auditory information processing. -

Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G
Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G. Jamieson, M.D. Weill Cornell Medicine, New York, NY The patient who presents with either acute or subacute confusion, in the absence of a clearly defined speech disorder and focality on neurological examination that would indicate an underlying mass lesion, needs to be evaluated for a multitude of neurological conditions. Many of the conditions that produce the recent onset of alteration in mental status, that ranges from mild confusion to florid delirium, may be due to infectious or inflammatory conditions that warrant acute intervention such as antimicrobial drugs, steroids or plasma exchange. However, some patients with recent onset of confusion have an underlying toxic-metabolic disorders indicating a specific diagnosis with need for appropriate treatment. The clinical presentations of some patients may indicate the diagnosis (e.g. hypoglycemia, chronic alcoholism) while the imaging patterns must be recognized to make the diagnosis in other patients. Toxic-metabolic disorders constitute a group of diseases and syndromes with diverse causes and clinical presentations. Many toxic-metabolic disorders have no specific neuroimaging correlates, either at early clinical stages or when florid symptoms develop. However, some toxic-metabolic disorders have characteristic abnormalities on neuroimaging, as certain areas of the central nervous system appear particularly vulnerable to specific toxins and metabolic perturbations. Areas of particular vulnerability in the brain include: 1) areas of high-oxygen demand (e.g. basal ganglia, cerebellum, hippocampus), 2) the cerebral white matter and 3) the mid-brain. Brain areas of high-oxygen demand are particularly vulnerable to toxins that interfere with cellular respiratory metabolism. -

MR Imaging of Primary Trochlear Nerve Neoplasms
707 MR Imaging of Primary Trochlear Nerve Neoplasms Lindell R. Gentry 1 We present the clinical, anatomic, and MR imaging findings in six patients with seven Rahul C. Mehta 1 primary trochlear nerve neoplasms, as well as the MR and clinical criteria that serve to Richard E. Appen2 establish the diagnosis of these rare cranial nerve neoplasms. Three patients had a Joel M. Weinstein2 history of neurofibromatosis and five patients had clinical evidence of a trochlear nerve palsy. Six of seven neoplasms produced localized, fusiform enlargement of the proximal cisternal segments of the trochlear nerves. The lesions that were visible on noncontrast MR scans (T1-, T2-, and proton density-weighted) had signal intensities that were virtually identical to normal brain parenchyma. All lesions showed intense, homogeneous enhancement on contrast-enhanced scans. Contrast-enhanced imaging was necessary for the detection of five of seven lesions and greatly increased the value of the MR study in all six patients. AJNR 12:707-713, July/August 1991; AJR 157: September 1991 Primary neoplasms arising from pure motor cranial nerves such as the trochlear nerve are rare. To our knowledge just nine primary trochlear nerve neoplasms have been reported in the literature [1-7), only one of which was imaged with MR [1). Over the last 6 years, since we began to use MR as the primary imaging method for evaluating patients with cranial nerve palsies , we have noticed a striking increase in the number of primary trochlear nerve neoplasms over those encountered during the CT era. We believe this is due to a much greater sensitivity of MR in detecting these typically small lesions. -

DR. Sanaa Alshaarawy
By DR. Sanaa Alshaarawy 1 By the end of the lecture, students will be able to : Distinguish the internal structure of the components of the brain stem in different levels and the specific criteria of each level. 1. Medulla oblongata (closed, mid and open medulla) 2. Pons (caudal, mid “Trigeminal level” and rostral). 3. Mid brain ( superior and inferior colliculi). Describe the Reticular formation (structure, function and pathway) being an important content of the brain stem. 2 1. Traversed by the Central Canal. Motor Decussation*. Spinal Nucleus of Trigeminal (Trigeminal sensory nucleus)* : ➢ It is a larger sensory T.S of Caudal part of M.O. nucleus. ➢ It is the brain stem continuation of the Substantia Gelatinosa of spinal cord 3 The Nucleus Extends : Through the whole length of the brain stem and upper segments of spinal cord. It lies in all levels of M.O, medial to the spinal tract of the trigeminal. It receives pain and temperature from face, forehead. Its tract present in all levels of M.O. is formed of descending fibers that terminate in the trigeminal nucleus. 4 It is Motor Decussation. Formed by pyramidal fibers, (75-90%) cross to the opposite side They descend in the Decuss- = crossing lateral white column of the spinal cord as the lateral corticospinal tract. The uncrossed fibers form the ventral corticospinal tract. 5 Traversed by Central Canal. Larger size Gracile & Cuneate nuclei, concerned with proprioceptive deep sensations of the body. Axons of Gracile & Cuneate nuclei form the internal arcuate fibers; decussating forming Sensory Decussation. Pyramids are prominent ventrally. 6 Formed by the crossed internal arcuate fibers Medial Leminiscus: Composed of the ascending internal arcuate fibers after their crossing.