Functional Significance of the Ventral and Lateral Funiculi in the Frog's Spinal Cord
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Major Motor & Sensory Projections
Major Motor & Sensory Projections Neuroanatomy > Brainstem > Brainstem MAJOR MOTOR & SENSORY PROJECTIONS  FULL TEXT OVERVIEW • Here, we will learn a consolidated view of the major descending (motor) and ascending (sensory) pathways from the cerebrum through the brainstem into the spinal cord. • Start a table, specify that we will learn about the following major pathway projections: Motor • Corticospinal tract (CST) • Corticobulbar (aka corticonuclear) tract Sensory • Posterior column/medial lemniscus • Anterolateral system (spinothalamic) tract • Trigeminothalamic tract MOTOR PATHWAYS Anatomical Structures Begin with the motor pathways. • Label the superior/inferior axes. • Draw a coronal view of the right brain. • Then, the brainstem. • The upper cervical spinal cord. 1 / 5 • And the lumbar cord. Innervation • Start another table. Write that for the motor fibers: • The corticospinal tract fibers innervate the body via spinal motor neurons. - Specify that the lateral CST innervates distal musculature for fine motor movements - Whereas the anterior CST innervates proximal musculature for gross motor movements. • The corticobulbar fibers (aka corticonuclear fibers) innervate the face via the CNs. Projections • Now, demarcate the internal capsule deep within the cerebrum – the motor fibers consolidate here before entering the ipsilateral cerebral peduncle in the midbrain. • Next, draw the twisting descent of each fiber group through the subcortical white matter. • Show that the facial fibers [RED] emerge from the lateral convexity, descend medially and, generally, decussate to synapse on different cranial nerve nuclei throughout their descent. • Then, show that the leg fibers [GREEN] emerge paracentrally, descend laterally through the brainstem into the ipsilateral medullary pyramid. • Show that the arm fibers [BLUE] emerge from the upper convexity, descend in between the facial and leg fibers, medial to the leg fibers through the brainstem into the ipsilateral medullary pyramid. -

High-Resolution MR Imaging of the Cadaveric Human Spinal Cord: Normal Anatomy
3 High-Resolution MR Imaging of the Cadaveric Human Spinal Cord: Normal Anatomy M. D. Solsberg1 The purpose of this study was to demonstrate the regional MR anatomy of a normal 1 2 C. Lemaire • human spinal cord under near optimal conditions. A spinal cord and meninges were L. Resch3 excised and segments from the cervical (C6), thoracic (T6), lumbar (L3), and sacral/ D. G. Potts1 cauda equina regions were examined on a 2-T MR system. By using a 2.5 x 2.0 em solenoid coil and a multislice spin-echo sequence, we achieved a resolution of 58 llm in the readout direction and 117 llm in the phase-encode direction. Histological sections corresponding to the areas imaged by MR were retained and treated with stains that demonstrated the distributions of collagen (hematoxylin, phloxine, saffron), myelin (Luxol fast bluefH and E), or neuritic processes (Bielschowsky's). Subarachnoid, vas cular, white matter, and gray matter structures were demonstrated by MR and light microscopy. The resulting MR images and photomicrographs were correlated. Different signal intensities were observed in the gracile and cuneate fasciculi, and these differ ences were similar to the pattern seen with the myelin stain. Decreased signal intensity was present in the region of the spinocerebellar tracts. The anatomic detail demonstrated by this study was clearly superior to that shown by clinical MR examinations. AJNR 11:3-7, January/February 1990 Because MR imaging of the human spinal cord demonstrates clearly the gray and white matter structures [1-5], this technique is useful for diagnosing variou s cord abnormalities, such as syringomyelia, intramedullary tumors, cord atrophy demyelination , cord cysts, and vascular malformations. -

White Matter Anatomy: What the Radiologist Needs to Know
White Matter Anatomy What the Radiologist Needs to Know Victor Wycoco, MBBS, FRANZCRa, Manohar Shroff, MD, DABR, FRCPCa,*, Sniya Sudhakar, MBBS, DNB, MDb, Wayne Lee, MSca KEYWORDS Diffusion tensor imaging (DTI) White matter tracts Projection fibers Association Fibers Commissural fibers KEY POINTS Diffusion tensor imaging (DTI) has emerged as an excellent tool for in vivo demonstration of white matter microstructure and has revolutionized our understanding of the same. Information on normal connectivity and relations of different white matter networks and their role in different disease conditions is still evolving. Evidence is mounting on causal relations of abnormal white matter microstructure and connectivity in a wide range of pediatric neurocognitive and white matter diseases. Hence there is a pressing need for every neuroradiologist to acquire a strong basic knowledge of white matter anatomy and to make an effort to apply this knowledge in routine reporting. INTRODUCTION (Fig. 1). However, the use of specific DTI sequences provides far more detailed and clini- DTI has allowed in vivo demonstration of axonal cally useful information. architecture and connectivity. This technique has set the stage for numerous studies on normal and abnormal connectivity and their role in devel- DIFFUSION TENSOR IMAGING: THE BASICS opmental and acquired disorders. Referencing established white matter anatomy, DTI atlases, Using appropriate magnetic field gradients, and neuroanatomical descriptions, this article diffusion-weighted sequences can be used to summarizes the major white matter anatomy and detect the motion of the water molecules to and related structures relevant to the clinical neurora- from cells. This free movement of the water mole- diologist in daily practice. -

The Spinal Cord
1/2/2016 The Spinal Cord • Continuation of CNS inferior to foramen magnum The Nervous System – Simpler than the brain – Conducts impulses to and from brain • Two way conduction pathway Spinal Cord – Reflex actions The Spinal Cord CCeerrvviiccaallCervical CCeerrvviiccaallCervical spinal nerves • Passes through vertebral canal enlargement – Foramen magnum L2 DDuurraaDura aannddand (a) The spinal cord and its nerve arachnoid roots, with the bony vertebral TThhoorraacciiccThoracic – Conus medullaris = tapered end of the cord mmaatteerrmater arches removed. The dura mater spinal nerves and arachnoid mater are cut – LLuummbbaarrLumbar Filum terminale = anchors the cord open and reflected laterally. enlargement – Cauda equina = bundle of lower spinal nerves CCoonnuussConus medullaris LLuummbbaarrLumbar CCaauuddaaCauda spinal nerves eeqquuiinnaaequina FFiilluummFilum terminale SSaaccrraallSacral spinal nerves The Spinal Cord CCeerrvviiccaallCervical CCeerrvviiccaallCervical spinal nerves • Spinal nerves enlargement – 31 pairs DDuurraaDura aannddand arachnoid TThhoorraacciiccThoracic mmaatteerrmater • Cervical and lumbar enlargements spinal nerves – Nerves serving the upper & lower limbs emerge LLuummbbaarrLumbar enlargement here CCoonnuussConus medullaris LLuummbbaarrLumbar CCaauuddaaCauda spinal nerves eeqquuiinnaaequina FFiilluummFilum (a) The spinal cord and its nerve terminale SSaaccrraallSacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a -

Pial Surface CSF-Contacting Texture, Subpial and Funicular Plexus in the Thoracic Spinal Cord in Monkey: NADPH Diaphorase Histological Configuration
bioRxiv preprint doi: https://doi.org/10.1101/2020.01.30.927509; this version posted January 31, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Pial surface CSF-contacting texture, subpial and funicular plexus in the thoracic spinal cord in monkey: NADPH diaphorase histological configuration Yinhua Li1#, Wei Hou1#, Yunge Jia1#, Xiaoxin Wen1, Chenxu Rao1, Ximeng Xu1, Zichun Wei1, Lu Bai1, Huibing Tan1,2* 1 Department of Anatomy, Jinzhou Medical University, Jinzhou, Liaoning 121001, China 2 Department of Neurobiology, Jinzhou Medical University, Jinzhou, Liaoning 121001, China Running title: Funicular neurons in monkey thoracic spinal cord * Correspondence: Department of Anatomy, Jinzhou Medical University, Jinzhou, Liaoning 121001, China, [email protected] # The first three authors make equal contributions to this work. Abstract In spinal cord, white matter is distinguished from grey matter in that it contains ascending and descending axonal tracts. While grey matter gets concentrated with neuronal cell bodies. Notable cell bodies and sensory modality of cerebral spinal fluid (CSF) in white matter are still elusive in certain segment of the spinal cord. Monkey Spinal cord was examined by NADPH diaphorase (NADPH-d) histochemistry. We found that NADPH-d positive neurons clustered and featured flat plane in mediolateral funiculus in caudal thoracic and rostral lumber spinal cord, especially evident in the horizontal sections. Majority of NADPH-d funicular neurons were relatively large size and moderately- or lightly-stained neurons. -

The Physiology and Processing of Pain a Review
AACN Clinical Issues Volume 16, Number 3, pp. 277–290 C 2005, AACN The Physiology and Processing of Pain A Review Cynthia L. Renn, RN, PhD, ACNP; Susan G. Dorsey, RN, PhD 6 visits to a healthcare provider4 and the American Academy of Pain Management Despite the many advances in our reports5 that uncontrolled pain is at epidemic understanding of the mechanisms proportions, with 50 million Americans suf- underlying pain processing, pain fering from some form of chronic pain and continues to be a major healthcare another 25 million experiencing acute pain problem in the United States. Each day, caused by accident or surgical procedures millions of Americans are affected by both each year. Studies6–8 estimate that 70 million acute and chronic pain conditions, costing visits to healthcare providers were motivated in excess of $100 billion for by pain and that 4.9 million people visited a treatment-related costs and lost work healthcare provider for treatment of chronic productivity. Thus, it is imperative that pain, all at an estimated cost exceeding $100 better treatment strategies be developed. billion. Further, pain patients and their fami- One step toward improving pain lies suffer from intangible costs related to the management is through increased pain, such as decreased quality of life, de- knowledge of pain physiology. Within the pression, and interpersonal stresses.9 nervous system, there are several Pain not only affects patients and their pathways that transmit information about families, but also society and the economy pain from the periphery to the brain. There as well. Beyond the cost of medical treat- is also a network of pathways that carry ment, society bears the costs of increased modulatory signals from the brain and healthcare utilization and lost productivity brainstem that alter the incoming flow of by patients in pain. -

INFORMATION to USERS the Most Advanced Technology Has Been Used to Photo Graph and Reproduce This Manuscript from the Microfilm Master
INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. Also, if unauthorized copyright material had to be removed, a note will indicate the deletion. Oversize materials (e.g., maps, drawings, charts) are re produced by sectioning the original, beginning at the upper left-hand corner and continuing from left to right in equal sections with small overlaps. Each original is also photographed in one exposure and is included in reduced form at the back of the book. These are also available as one exposure on a standard 35mm slide or as a 17" x 23" black and white photographic print for an additional charge. Photographs included in the original manuscript have been reproduced xerographically in this copy. Higher quality 6" x 9" black and white photographic prints are available for any photographs or illustrations appearing in this copy for an additional charge. Contact UMI directly to order. University Microfilms International A Bell & Howell Information Company 300 North Zeeb Road, Ann Arbor, Ml 48106-1346 USA 313/761-4700 800/521-0600 Order Number 8913623 Analyses of the response of dorsal root afferents following dorsal funiculus lesions and an examination of the sacral parasympathetic nucleus in amphibians Campbell, H. -
Lab 2. Medulla A
Lab 2. Medulla A. Lesion Lessons Lesion 3.1. Mike Rowmeter i) Location: ii) Signs/symptoms: iii) Cause: Lesion 3.2. Anne Chovee i) Location: ii) Signs/symptoms: iii) Cause: Medical Neuroscience 3– Spinomedullary junction. Locate and note the following: • the enlarged substantia gelatinosa of the dorsal horn is a caudal extension of the spinal tri- geminal nucleus. • the spinal trigeminal tract is located superficially to the nucleus and is made up ofprimary trigeminal afferent fibers that entered the brain stem in the pons and then descended to this level. •MLF (medial longitudinal fasciculus) is often considered to include these tracts: – reticulospinal – tectospinal Label these structures on – medial vestibulospinal Figure 1. • fasciculus gracilis • fasciculus cuneatus hypothalamic autonomic tract • spinal trigeminal nuc. – also known as “descending sympathetics” • spinal tract of V • dorsal spinocerebellar tr. • descends diffusely in the lateral brain stem • ventral spinocerebellar tr • projects to the intermediolateral nucleus of the • spinothalamic tr. spinal cord • rubrospinal tract • lesion –> Horner’s syndrome (Ptosis, miosis, • lat. corticospinal tr. anhydrosis, and enopthalmos • ant. corticospinal tr. • vestibulospinal tr. Fig 1. Spinomedullary junction. From G. Jelg- ersma, Tabula 106, 1931 3– Loyola University Medullary Level of the Pyramidal (Motor) Decussation Locate and note the following: Question classic • pyramidal decussation – obvious in the ventral midline. Which types of sensory – pyramidal tract - fibers arise in cerebral cortex, descend to the lower medulla, modalities are con- and cross here to the contralateral spinal cord where they form the lateral veyed by the dorsal corticospinal tract that runs in the lateral funiculus. columns? • spinal trigeminal nucleus replaces the dorsal horn in function. -

Glossary of Neuroanatomical Terms and Eponyms
Copyright © 2008 by J. A. Kiernan Glossary of Neuroanatomical Terms and Eponyms The standard Latin forms of anatomical names are anglicized wherever this is possible without loss of euphony. Most anatomical terms have Latin origins, and most names related to diseases are derived from Greek words. Abbreviations Eng. English; Fr. French; Ger. German; Gr. Greek; L. Latin; O.E. Old English Neuroanatomical terms Abducens. L. ab, from + ducens, leading. Abducens (or abducent) nerve supplies the muscle that moves the direction of gaze away from the midline. Abulia. Gr. a, without + boule, will. A loss of will power. (Also spelled aboulia.) Accumbens. L. reclining. The nucleus accumbens is the ventral part of the head of the caudate nucleus, anterior and ventral to the anterior limb of the corpud callosum. Adenohypophysis. Gr. aden, gland + hypophysis (which see). The part of the pituitary gland derived from the pharyngeal endoderm (Rathke's pouch). Its largest part is the anterior lobe of the pituitary gland. Adiadochokinesia. a, neg. + Gr. diadochos, succeeding + kinesis, movement. Inability to perform rapidly alternating movements. Also called dysdiadochokinesia. Ageusia. Gr. a, without + geuein, to taste. Loss of the sense of taste. Agnosia. a, neg. + Gr. gnosis, knowledge. Lack of ability to recognize the significance of sensory stimuli (auditory, visual, tactile, etc. agnosia). Agraphia. a, neg. + Gr. graphein, to write. Inability to express thoughts in writing owing to a central lesion. Akinesia. a, neg. + Gr. kinesis, movement. Lack of spontaneous movement, as seen in Parkinson's disease. Ala cinerea. L. wing + cinereus, ashen-hued. Vagal triangle in floor of fourth ventricle. Alexia. -
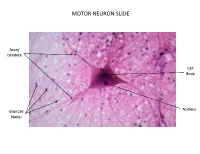
Motor Neuron Slide
MOTOR NEURON SLIDE Axon/ Dendrite s Cell Body Nucleus Glial Cell Nuclei ________________ _______ _______ _______ NEURON MODEL Dendrites Schwann Cells Axon Cell Body Axon Hillock Nissl Bodies (special name Nucleus for rough e.r.) NEURON MODEL Myelin Sheath Myelin Sheath (layers inside (layers inside each schwann each schwann cell) cell) Neurilemma (outermost layer of schwann cell) Node of Ranvier Endoneurium (gaps between (connective tissue schwann cells) around axon) NEURON MODEL _______ _______ _______ _______ _______ _______ ______ ______ _ _______ NEURON MODEL _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ _______ PERIPHERAL NERVE SLIDE Fasciculi Axon Perineurium Myelin Endorineurium ______________________ Cross Section _______ _______ _______ _______ _______ PERIPHERAL NERVE SLIDE (longitudinal) Node of Myelin Ranvier Axon ________________________ _______ _______ _______ _______ BRAIN MODEL Right Cerebral Left Cerebral Hemisphere Hemisphere Sulci Gyri (grooves (bumps on in brain) brain) Longitudinal Frontal Median Anterior Lobe Fissure View Temporal Lobe Lateral Sulcus Parietal Occipital Lobes Lobe s Posterior View BRAIN MODEL Pituitary Optic Gland Chiasma Mammillary Pons Body Cerebellum Medulla Oblongata Cerebrum Gyri Sulci (grooves (bumps on brain) in brain) Interthalamic Fornix Adhesion Choroid Plexus Thalamus Septum (within the 3rd Pellucidum Ventricle) Hypot halamus Pineal Gland (within the 3rd Ventricle) Cerebral Aqueduct (cavity that leads to the 4th Ventricle)