Neuroanatomy ©
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience
CNs 5, 7, 9, 10 - Pharnygeal Arch Set - Motor USMLE, Limited Edition > Neuroscience > Neuroscience PHARYNGEAL ARCH SET, CNS 5, 7, 9, 10 • They are derived from the pharyngeal (aka branchial) arches • They have special motor and autonomic motor functions CRANIAL NERVES EXIT FROM THE BRAINSTEM CN 5, the trigeminal nerve exits the mid/lower pons.* CN 7, the facial nerve exits the pontomedullary junction.* CN 9, the glossopharyngeal nerve exits the lateral medulla.* CN 10, the vagus nerve exits the lateral medulla.* CRANIAL NERVE NUCLEI AT BRAINSTEM LEVELS Midbrain • The motor trigeminal nucleus of CN 5. Nerve Path: • The motor division of the trigeminal nerve passes laterally to enter cerebellopontine angle cistern. Pons • The facial nucleus of CN 7. • The superior salivatory nucleus of CN 7. Nerve Path: • CN 7 sweeps over the abducens nucleus as it exits the brainstem laterally in an internal genu, which generates a small bump in the floor of the fourth ventricle: the facial colliculus • Fibers emanate from the superior salivatory nucleus, as well. Medulla • The dorsal motor nucleus of the vagus, CN 10 • The inferior salivatory nucleus, CN 9 1 / 3 • The nucleus ambiguus, CNs 9 and 10. Nerve Paths: • CNs 9 and 10 exit the medulla laterally through the post-olivary sulcus to enter the cerebellomedullary cistern. THE TRIGEMINAL NERVE, CN 5  • The motor division of the trigeminal nerve innervates the muscles of mastication • It passes ventrolaterally through the cerebellopontine angle cistern and exits through foramen ovale as part of the mandibular division (CN 5[3]). Clinical Correlation - Trigeminal Neuropathy THE FACIAL NERVE, CN 7  • The facial nucleus innervates the muscles of facial expression • It spans from the lower pons to the pontomedullary junction. -

Functional Imaging of the Deep Cerebellar Nuclei: a Review
Cerebellum (2010) 9:22–28 DOI 10.1007/s12311-009-0119-3 Functional Imaging of the Deep Cerebellar Nuclei: A Review Christophe Habas Published online: 10 June 2009 # Springer Science + Business Media, LLC 2009 Abstract The present mini-review focused on functional and climbing fibers derived from the bulbar olivary nuclei. imaging of human deep cerebellar nuclei, mainly the The mossy-fiber influence on DCN firing appears to be dentate nucleus. Although these nuclei represent the unique weaker than the climbing-fiber influence. Despite the output channel of the cerebellum, few data are available pivotal role of these nuclei, only very few results are concerning their functional role. However, the dentate available for functional imaging of the DCN, including nucleus has been shown to participate in a widespread PET scan and MRI techniques, and most of these data functional network including sensorimotor and associative concern the DN. This lack of data is due to a number of cortices, striatum, hypothalamus, and thalamus, and plays a reasons. minor role in motor execution and a major role in First, the human DCN mainly comprise the large, sensorimotor coordination and learning, and cognition. widespread, and easily identifiable DN which has a marked The dentate nucleus appears to be predominantly involved low-intensity signal on MRI T2*-weighted sequences and in conjunction with the neocerebellum in executive and is clearly distinguished from the adjacent cortical structures. affective networks devoted, at least, to attention, working In contrast, FN and GEN are very thin and are both located memory, procedural reasoning, and salience detection. very close to the gray matter of lobules VIII and IX, while these nuclei are situated on the medial aspect of the DN. -

The Nerve Lesion in the Carpal Tunnel Syndrome
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.39.7.615 on 1 July 1976. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1976, 39, 615-626 The nerve lesion in the carpal tunnel syndrome SYDNEY SUNDERLAND From the Department of Experimental Neurology, University of Melbourne, Parkville, Victoria, Australia SYNOPSIS The relative roles of pressure deformation and ischaemia in the production of compres- sion nerve lesions remain a controversial issue. This paper concerns the genesis of the structural changes which follow compression of the median nerve in the carpal tunnel. The initial lesion is an intrafunicular anoxia caused by obstruction to the venous return from the funiculi as the result of increased pressure in the tunnel. This leads to intrafunicular oedema and an increase in intrafunicular pressure which imperil and finally destroy nerve fibres by impairing their blood supply and by compression. The final outcome is the fibrous tissue replacement of the contents of the funiculi. Protected by copyright. In 1862 Waller described the motor, vasomotor, (Gasser, 1935; Allen, 1938; Bentley and Schlapp, and sensory changes which followed the com- 1943; Richards, 1951; Moldaver, 1954; Gelfan pression of nerves in his own arm. His account and Tarlov, 1956). carried no reference to the mechanism In the 1940s Weiss and his associates (Weiss, responsible for blocking conduction in the nerve 1943, 1944; Weiss and Davis, 1943; Weiss and fibres, presumably because he regarded it as Hiscoe, 1948), who were primarily concerned obvious that pressure was the offending agent. with the technical problem of uniting severed Interest in the effects of nerve compression nerves, observed that a divided nerve enclosed was not renewed until the 1920s when cuff or in a tightly fitting arterial sleeve became swollen tourniquet compression was used to produce a proximal and distal to the constriction. -

Quantitative Analysis of Axon Collaterals of Single Pyramidal Cells
Yang et al. BMC Neurosci (2017) 18:25 DOI 10.1186/s12868-017-0342-7 BMC Neuroscience RESEARCH ARTICLE Open Access Quantitative analysis of axon collaterals of single pyramidal cells of the anterior piriform cortex of the guinea pig Junli Yang1,2*, Gerhard Litscher1,3* , Zhongren Sun1*, Qiang Tang1, Kiyoshi Kishi2, Satoko Oda2, Masaaki Takayanagi2, Zemin Sheng1,4, Yang Liu1, Wenhai Guo1, Ting Zhang1, Lu Wang1,3, Ingrid Gaischek3, Daniela Litscher3, Irmgard Th. Lippe5 and Masaru Kuroda2 Abstract Background: The role of the piriform cortex (PC) in olfactory information processing remains largely unknown. The anterior part of the piriform cortex (APC) has been the focus of cortical-level studies of olfactory coding, and asso- ciative processes have attracted considerable attention as an important part in odor discrimination and olfactory information processing. Associational connections of pyramidal cells in the guinea pig APC were studied by direct visualization of axons stained and quantitatively analyzed by intracellular biocytin injection in vivo. Results: The observations illustrated that axon collaterals of the individual cells were widely and spatially distrib- uted within the PC, and sometimes also showed a long associational projection to the olfactory bulb (OB). The data showed that long associational axons were both rostrally and caudally directed throughout the PC, and the intrinsic associational fibers of pyramidal cells in the APC are omnidirectional connections in the PC. Within the PC, associa- tional axons typically followed rather linear trajectories and irregular bouton distributions. Quantitative data of the axon collaterals of two pyramidal cells in the APC showed that the average length of axonal collaterals was 101 mm, out of which 79 mm (78% of total length) were distributed in the PC. -

The Distribution of Immune Cells in the Uveal Tract of the Normal Eye
THE DISTRIBUTION OF IMMUNE CELLS IN THE UVEAL TRACT OF THE NORMAL EYE PAUL G. McMENAMIN Perth, Western Australia SUMMARY function of these cells in the normal iris, ciliary body Inflammatory and immune-mediated diseases of the and choroid. The role of such cell types in ocular eye are not purely the consequence of infiltrating inflammation, which will be discussed by other inflammatory cells but may be initiated or propagated authors in this issue, is not the major focus of this by immune cells which are resident or trafficking review; however, a few issues will be briefly through the normal eye. The uveal tract in particular considered where appropriate. is the major site of many such cells, including resident tissue macro phages, dendritic cells and mast cells. This MACRO PHAGES review considers the distribution and location of these and other cells in the iris, ciliary body and choroid in Mononuclear phagocytes arise from bone marrow the normal eye. The uveal tract contains rich networks precursors and after a brief journey in the blood as of both resident macrophages and MHe class 11+ monocytes immigrate into tissues to become macro dendritic cells. The latter appear strategically located to phages. In their mature form they are widely act as sentinels for capturing and sampling blood-borne distributed throughout the body. Macrophages are and intraocular antigens. Large numbers of mast cells professional phagocytes and play a pivotal role as are present in the choroid of most species but are effector cells in cell-mediated immunity and inflam virtually absent from the anterior uvea in many mation.1 In addition, due to their active secretion of a laboratory animals; however, the human iris does range of important biologically active molecules such contain mast cells. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -

A Cyclops and a Synotus by K
J Neurol Psychopathol: first published as 10.1136/jnnp.s1-17.65.48 on 1 July 1936. Downloaded from 48 ORIGINAL PAPERS A CYCLOPS AND A SYNOTUS BY K. H. BOUMAN, AMSTERDAM, AND V. W. D. SCHENK, TiH HAGUE INTRODUCTION ONLY a small number of cases of cyclopia in human beings and mammals have been minutely examined. The number becomes still smaller if a more or less complete microscopic investigation of the central nervous system is stipulated. It is really only the cases of Davidson Black and Winkler and perhaps that of Naegli which answer this requirement. In contrast therewith there is an abundance of experimental studies in this field in urodela and other lower classes of animals. For all that, unanimity does not by any means prevail here, although the Protected by copyright. views of Stockard and his followers-who held that the first determination of the eye lay unpaired in the median line-and those of Spemann-who pointed to a paired rudiment from the outset, which views were originally diametrically opposed-appear to have drawn somewhat nearer to each other in recent years. Woerdeman, for instance, found that the paired rudiment of the eye shifts its position laterally downwards very early (when the folds of the medullary plate become visible) and he rightly says that this is not the same as Stockard's lateral growth of an unpaired eye rudiment. Yet, by saying this, he admits certain changes and growth conditions to which Fischel, for instance, did not do full justice. E. Manchot, on the other hand, who defends Stockard's views, admits that between the two regions of the eye http://jnnp.bmj.com/ rudiment there must be a tract of brain tissue (lamina terminalis and regio chiasmatica). -

Ciliary Zonule Sclera (Suspensory Choroid Ligament)
ACTIVITIES Complete Diagrams PNS 18 and 19 Complete PNS 23 Worksheet 3 #1 only Complete PNS 24 Practice Quiz THE SPECIAL SENSES Introduction Vision RECEPTORS Structures designed to respond to stimuli Variable complexity GENERAL PROPERTIES OF RECEPTORS Transducers Receptor potential Generator potential GENERAL PROPERTIES OF RECEPTORS Stimulus causing receptor potentials Generator potential in afferent neuron Nerve impulse SENSATION AND PERCEPTION Stimulatory input Conscious level = perception Awareness = sensation GENERAL PROPERTIES OF RECEPTORS Information conveyed by receptors . Modality . Location . Intensity . Duration ADAPTATION Reduction in rate of impulse transmission when stimulus is prolonged CLASSIFICATION OF RECEPTORS Stimulus Modality . Chemoreceptors . Thermoreceptors . Nociceptors . Mechanoreceptors . Photoreceptors CLASSIFICATION OF RECEPTORS Origin of stimuli . Exteroceptors . Interoceptors . Proprioceptors SPECIAL SENSES Vision Hearing Olfaction Gustation VISION INTRODUCTION 70% of all sensory receptors are in the eye Nearly half of the cerebral cortex is involved in processing visual information Optic nerve is one of body’s largest nerve tracts VISION INTRODUCTION The eye is a photoreceptor organ Refraction Conversion (transduction) of light into AP’s Information is interpreted in cerebral cortex Eyebrow Eyelid Eyelashes Site where conjunctiva merges with cornea Palpebral fissure Lateral commissure Eyelid Medial commissure (a) Surface anatomy of the right eye Figure 15.1a Orbicularis oculi muscle -
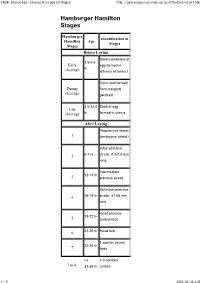
Hamburger Hamilton Stages
UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm Hamburger Hamilton Stages Hamburger Identification of Hamilton Age Stages Stages Before Laying Shell membrane of 3.5-4.5 Early egg formed in hr cleavage isthmus of oviduct Germ wall formed During from marginal cleavage periblast 4.5-24.0 Shell of egg Late cleavage hr formed in uterus After Laying Preprimitive streak 1 (embryonic shield) Initial primitive 2 6-7 hr streak, 0.3-0.5 mm long Intermediate 12-13 hr 3 primitive streak Definitive primitive 4 18-19 hr streak, ±1.88 mm long Head process 19-22 hr 5 (notochord) 6 23-25 hr Head fold 1 somite; neural 23-26 hr 7 folds ca. 1-3 somites; 7 to 8- 23-26 hr coelom 1 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm 4 somites; blood 26-29 hr 8 islands 7 somites; primary 29-33 hr 9 optic vesicles 8-9 somites; 9+ to 10- ca. 33 hr anterior amniotic fold 10 somites; 3 10 33-38 hr primary brain vesicles 13 somites; 5 11 40-45 hr neuromeres of hindbrain 16 somites; 45-49 hr 12 telencephalon 19 somites; 13 48-52 hr atrioventricular canal ca. 20-21 somites; tail 13+ to 14- 50-52 hr bud 22 somites; trunk flexure; visceral 50-53 hr 14 arches I and II, clefts 1 and 2 23 somites; ca. premandibular 14+ to 15- 50-54 hr head cavities 24-27 somites; 15 50-55 hr visceral arch III, cleft 3 26-28 somites; 16 51-56 hr wing bud; posterior 2 / 5 2007/03/20 9:05 UNSW Embryology- Chicken Development Stages http://embryology.med.unsw.edu.au/OtherEmb/chick1.htm -

Deconstructing Spinal Interneurons, One Cell Type at a Time Mariano Ignacio Gabitto
Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy under the Executive Committee of the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2016 © 2016 Mariano Ignacio Gabitto All rights reserved ABSTRACT Deconstructing spinal interneurons, one cell type at a time Mariano Ignacio Gabitto Abstract Documenting the extent of cellular diversity is a critical step in defining the functional organization of the nervous system. In this context, we sought to develop statistical methods capable of revealing underlying cellular diversity given incomplete data sampling - a common problem in biological systems, where complete descriptions of cellular characteristics are rarely available. We devised a sparse Bayesian framework that infers cell type diversity from partial or incomplete transcription factor expression data. This framework appropriately handles estimation uncertainty, can incorporate multiple cellular characteristics, and can be used to optimize experimental design. We applied this framework to characterize a cardinal inhibitory population in the spinal cord. Animals generate movement by engaging spinal circuits that direct precise sequences of muscle contraction, but the identity and organizational logic of local interneurons that lie at the core of these circuits remain unresolved. By using our Sparse Bayesian approach, we showed that V1 interneurons, a major inhibitory population that controls motor output, fractionate into diverse subsets on the basis of the expression of nineteen transcription factors. Transcriptionally defined subsets exhibit highly structured spatial distributions with mediolateral and dorsoventral positional biases. These distinctions in settling position are largely predictive of patterns of input from sensory and motor neurons, arguing that settling position is a determinant of inhibitory microcircuit organization. -

Clones in the Chick Diencephalon Contain Multiple Cell Types and Siblings Are Widely Dispersed
Development 122, 65-78 (1996) 65 Printed in Great Britain © The Company of Biologists Limited 1996 DEV8292 Clones in the chick diencephalon contain multiple cell types and siblings are widely dispersed Jeffrey A. Golden1,2 and Constance L. Cepko1,3 1Department of Genetics, Harvard Medical School, 2Department of Pathology, Brigham and Women’s Hospital, and 3Howard Hughes Medical Institute, 200 Longwood Avenue, Boston, MA 02115, USA SUMMARY The thalamus, hypothalamus and epithalamus of the ver- clones dispersed in all directions, resulting in sibling cells tebrate central nervous system are derived from the populating multiple nuclei within the diencephalon. In embryonic diencephalon. These regions of the nervous addition, several distinctive patterns of dispersion were system function as major relays between the telencephalon observed. These included clones with siblings distributed and more caudal regions of the brain. Early in develop- bilaterally across the third ventricle, clones that originated ment, the diencephalon morphologically comprises distinct in the lateral ventricle, clones that crossed neuromeric units known as neuromeres or prosomeres. As development boundaries, and clones that crossed major boundaries of proceeds, multiple nuclei, the functional and anatomical the developing nervous system, such as the diencephalon units of the diencephalon, derive from the neuromeres. It and mesencephalon. These findings demonstrate that prog- was of interest to determine whether progenitors in the enitor cells in the diencephalon are multipotent and that diencephalon give rise to daughters that cross nuclear or their daughters can become widely dispersed. neuromeric boundaries. To this end, a highly complex retroviral library was used to infect diencephalic progeni- tors. Retrovirally marked clones were found to contain Key words: cell lineage, central nervous system, diencephalon, neurons, glia and occasionally radial glia. -

Isolated Relative Afferent Pupillary Defect Secondary to Contralateral Midbrain Compression
OBSERVATION Isolated Relative Afferent Pupillary Defect Secondary to Contralateral Midbrain Compression Cheun Ju Chen, MD; Mia Scheufele, MD; Maushmi Sheth, MD; Amir Torabi, MD; Nick Hogan, MD, PhD; Elliot M. Frohman, MD, PhD Background: Relative afferent pupillary defects are typi- accounts for the relative afferent pupillary defect con- cally related to ipsilateral lesions within the anterior vi- tralateral to the described lesion. sual pathways. Result: Magnetic resonance imaging of the brain revealed a pineal tumor compressing the right rostral midbrain. Objective: To describe a patient who had a workup for headache and was found to have an isolated left relative Conclusion: While rare, a relative afferent pupillary de- afferent pupillary defect without any other neurological fect can occasionally occur secondary to lesions in the findings. postchiasmal pathways. In these circumstances, the pu- pillary defect will be observed to be contralateral to the Design: We review the neuroanatomy of the pupil- side of the lesion. lary light reflex pathway and emphasize the nasotem- poral bias of decussating fiber projections, which Arch Neurol. 2004;61:1451-1453 RELATIVE AFFERENT PUPIL- though retinal fibers concerned with this lary defect (RAPD) is char- reflex transmit information to both the ip- acterized by pupillary dila- silateral and contralateral midbrain, there tion upon illuminating the is a slight crossing bias, with about 53% of eye during the swinging the fibers crossing in the optic chiasm Aflashlight test. The presence of this sign sig- (chiefly derived from the nasal retina) and nifies an abnormality in the transmission 47% remaining ipsilateral. This anatomi- of light information within the pupillary cal organization of the pupillary constric- light constrictor pathway from the retina tor pathway results in the possibility of pro- to the rostral midbrain circuitry involved ducing an RAPD during illumination of the in this reflex.