Corticospinal Tract (CST) Reconstruction Based on Fiber
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

A Network of Genetic Repression and Derepression Specifies Projection
A network of genetic repression and derepression INAUGURAL ARTICLE specifies projection fates in the developing neocortex Karpagam Srinivasana,1, Dino P. Leonea, Rosalie K. Batesona, Gergana Dobrevab, Yoshinori Kohwic, Terumi Kohwi-Shigematsuc, Rudolf Grosschedlb, and Susan K. McConnella,2 aDepartment of Biology, Stanford University, Stanford, CA 94305; bMax-Planck Institute of Immunobiology and Epigenetics, 79108 Freiburg, Germany; and cLawrence Berkeley National Laboratory, Berkeley, CA 94720 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2011. Contributed by Susan K. McConnell, September 28, 2012 (sent for review August 24, 2012) Neurons within each layer in the mammalian cortex have stereotypic which results in a suppression of corticothalamic and callosal projections. Four genes—Fezf2, Ctip2, Tbr1, and Satb2—regulate fates, respectively, and axon extension along the corticospinal these projection identities. These genes also interact with each tract (CST). Fezf2 mutant neurons fail to repress Satb2 and Tbr1, other, and it is unclear how these interactions shape the final pro- thus their axons cross the CC and/or innervate the thalamus jection identity. Here we show, by generating double mutants of inappropriately. Fezf2, Ctip2, and Satb2, that cortical neurons deploy a complex In callosal projection neurons, Satb2 represses the expression Ctip2 Bhlhb5 genetic switch that uses mutual repression to produce subcortical of and , leading to a repression of subcerebral fates. Satb2 Tbr1 or callosal projections. We discovered that Tbr1, EphA4, and Unc5H3 Interestingly, we found that promotes expression in fi upper layer callosal neurons, and Tbr1 expression in these neu- are critical downstream targets of Satb2 in callosal fate speci ca- fi tion. -
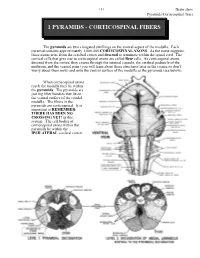
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Spinal Tracts.Pdf
Spinal Tracts Andreas Talgø Lie Illustrations by: Peder Olai Skjeflo Holman Previous material: Maja Solbakken Definitions to bring home Nerve Ganglion Neuron Nucleus Tract Collection neurons Collection of nerve A single cell Collection of Collection of axons that transmits cell bodies in the transmitting nerve cell bodies in traveling up or sensation or motor PNS, typically linked electrical impluses. the CNS, typically down the spinal impulses depending by synapses. linked by synapses. cord, depending on the function and Location: both on function destination. Location: PNS Location: CNS and destination. Location: PNS Location: CNS - does it matter? Tract - highway to pass anatomy exam? • Highway = Tract • Lane = Neuron • Car = Signal Slow... Fast!! Just to make sure... • Ipsilateral or contralateral • Ventral = anterior • Dorsal = posterior High yield points to understand • What does the tract transmit - Motor or sensory? If sensory: which sensation? • Where does the neurones synapse - 1.st, 2nd and 3rd order neron? Which ganglion/nuclei? • Where there are decussations - If there are any decussations at all? ASCENDING / SENSORY TRACTS Sensations * Temperature NOT transmitted by the tracts: * Pressure * Visualization * Pain * Audition * Fine touch * Olfaction * Crude touch * Gustation * Proprioception * Vibration Sensations Precise sensation Primitive sensation Fine touch Crude touch Pressure Pain Vibration Temperature Proprioception Other: sexual, itching, tickling Ascending tracts / Sensory tracts Dorsal coulmn Lat. Spinothalamic Ant. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -

Basal Ganglia & Cerebellum
1/2/2019 This power point is made available as an educational resource or study aid for your use only. This presentation may not be duplicated for others and should not be redistributed or posted anywhere on the internet or on any personal websites. Your use of this resource is with the acknowledgment and acceptance of those restrictions. Basal Ganglia & Cerebellum – a quick overview MHD-Neuroanatomy – Neuroscience Block Gregory Gruener, MD, MBA, MHPE Vice Dean for Education, SSOM Professor, Department of Neurology LUHS a member of Trinity Health Outcomes you want to accomplish Basal ganglia review Define and identify the major divisions of the basal ganglia List the major basal ganglia functional loops and roles List the components of the basal ganglia functional “circuitry” and associated neurotransmitters Describe the direct and indirect motor pathways and relevance/role of the substantia nigra compacta 1 1/2/2019 Basal Ganglia Terminology Striatum Caudate nucleus Nucleus accumbens Putamen Globus pallidus (pallidum) internal segment (GPi) external segment (GPe) Subthalamic nucleus Substantia nigra compact part (SNc) reticular part (SNr) Basal ganglia “circuitry” • BG have no major outputs to LMNs – Influence LMNs via the cerebral cortex • Input to striatum from cortex is excitatory – Glutamate is the neurotransmitter • Principal output from BG is via GPi + SNr – Output to thalamus, GABA is the neurotransmitter • Thalamocortical projections are excitatory – Concerned with motor “intention” • Balance of excitatory & inhibitory inputs to striatum, determine whether thalamus is suppressed BG circuits are parallel loops • Motor loop – Concerned with learned movements • Cognitive loop – Concerned with motor “intention” • Limbic loop – Emotional aspects of movements • Oculomotor loop – Concerned with voluntary saccades (fast eye-movements) 2 1/2/2019 Basal ganglia “circuitry” Cortex Striatum Thalamus GPi + SNr Nolte. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -

The Brain Stem Medulla Oblongata
Chapter 14 The Brain Stem Medulla Oblongata Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Central sulcus Parietal lobe • embryonic myelencephalon becomes Cingulate gyrus leaves medulla oblongata Corpus callosum Parieto–occipital sulcus Frontal lobe Occipital lobe • begins at foramen magnum of the skull Thalamus Habenula Anterior Epithalamus commissure Pineal gland • extends for about 3 cm rostrally and ends Hypothalamus Posterior commissure at a groove between the medulla and Optic chiasm Mammillary body pons Cerebral aqueduct Pituitary gland Fourth ventricle Temporal lobe • slightly wider than spinal cord Cerebellum Midbrain • pyramids – pair of external ridges on Pons Medulla anterior surface oblongata – resembles side-by-side baseball bats (a) • olive – a prominent bulge lateral to each pyramid • posteriorly, gracile and cuneate fasciculi of the spinal cord continue as two pair of ridges on the medulla • all nerve fibers connecting the brain to the spinal cord pass through the medulla • four pairs of cranial nerves begin or end in medulla - IX, X, XI, XII Medulla Oblongata Associated Functions • cardiac center – adjusts rate and force of heart • vasomotor center – adjusts blood vessel diameter • respiratory centers – control rate and depth of breathing • reflex centers – for coughing, sneezing, gagging, swallowing, vomiting, salivation, sweating, movements of tongue and head Medulla Oblongata Nucleus of hypoglossal nerve Fourth ventricle Gracile nucleus Nucleus of Cuneate nucleus vagus -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Interneuronal Activity in Reflex Pathways from Group II Muscle Afferents Is Monitored by Dorsal Spinocerebellar Tract Neurons in the Cat
The Journal of Neuroscience, April 2, 2008 • 28(14):3615–3622 • 3615 Behavioral/Systems/Cognitive Interneuronal Activity in Reflex Pathways from Group II Muscle Afferents Is Monitored by Dorsal Spinocerebellar Tract Neurons in the Cat Elzbieta Jankowska and Anna Puczynska Department of Physiology, Sahlgrenska Academy, Go¨teborg University, 405 30 Go¨teborg, Sweden The main aim of the study was to investigate whether group II muscle afferents contribute to the inhibition of dorsal spinocerebellar tract (DSCT) neurons and thereby modulate information provided by these neurons in the cat. In intracellular recordings, we found disynaptic IPSPs from group II afferents in the majority of DSCT neurons, most often in parallel with IPSPs evoked from group I afferents. In an attempt to identify interneurons that mediate these IPSPs, the second aim of the study, laminas IV–VII in midlumbar segments were searched for interneurons antidromically activated by stimuli applied within Clarke’s column. Such interneurons were found in regions in which focal field potentials were evoked by group I and II afferents, or ventral to them, and most were coexcited by these afferents. The input to these interneurons and their location indicate that they belonged to the previously identified population of premotor interneu- rons in disynaptic pathways between group I and II afferents and hindlimb motoneurons. The study leads thus to the conclusion that inhibitory actions of group II afferents on DSCT neurons are collateral to actions on motoneurons and that DSCT neurons monitor inhibitory actions of group II afferents on motoneurons as closely as they monitor actions of group I afferents. -

1. Lateral View of Lobes in Left Hemisphere TOPOGRAPHY
TOPOGRAPHY T1 Division of Cerebral Cortex into Lobes 1. Lateral View of Lobes in Left Hemisphere 2. Medial View of Lobes in Right Hemisphere PARIETAL PARIETAL LIMBIC FRONTAL FRONTAL INSULAR: buried OCCIPITAL OCCIPITAL in lateral fissure TEMPORAL TEMPORAL 3. Dorsal View of Lobes 4. Ventral View of Lobes PARIETAL TEMPORAL LIMBIC FRONTAL OCCIPITAL FRONTAL OCCIPITAL Comment: The cerebral lobes are arbitrary divisions of the cerebrum, taking their names, for the most part, from overlying bones. They are not functional subdivisions of the brain, but serve as a reference for locating specific functions within them. The anterior (rostral) end of the frontal lobe is referred to as the frontal pole. Similarly, the anterior end of the temporal lobe is the temporal pole, and the posterior end of the occipital lobe the occipital pole. TOPOGRAPHY T2 central sulcus central sulcus parietal frontal occipital lateral temporal lateral sulcus sulcus SUMMARY CARTOON: LOBES SUMMARY CARTOON: GYRI Lateral View of Left Hemisphere central sulcus postcentral superior parietal superior precentral gyrus gyrus lobule frontal intraparietal sulcus gyrus inferior parietal lobule: supramarginal and angular gyri middle frontal parieto-occipital sulcus gyrus incision for close-up below OP T preoccipital O notch inferior frontal cerebellum gyrus: O-orbital lateral T-triangular sulcus superior, middle and inferior temporal gyri OP-opercular Lateral View of Insula central sulcus cut surface corresponding to incision in above figure insula superior temporal gyrus Comment: Insula (insular gyri) exposed by removal of overlying opercula (“lids” of frontal and parietal cortex). TOPOGRAPHY T3 Language sites and arcuate fasciculus. MRI reconstruction from a volunteer. central sulcus supramarginal site (posterior Wernicke’s) Language sites (squares) approximated from electrical stimulation sites in patients undergoing operations for epilepsy or tumor removal (Ojeman and Berger).