Brainstem and Spinal Cord MRI Identifies Altered
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -
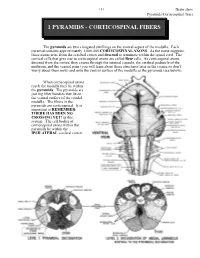
Corticospinal Fibers
151 Brain stem Pyramids/Corticospinal Tract 1 PYRAMIDS - CORTICOSPINAL FIBERS The pyramids are two elongated swellings on the ventral aspect of the medulla. Each pyramid contains approximately 1,000,000 CORTICOSPINAL AXONS. As the name suggests, these axons arise from the cerebral cortex and descend to terminate within the spinal cord. The cortical cells that give rise to corticospinal axons are called Betz cells. As corticospinal axons descend from the cortex, they course through the internal capsule, the cerebral peduncle of the midbrain, and the ventral pons (you will learn about these structures later in the course so don’t worry about them now) and onto the ventral surface of the medulla as the pyramids (see below). When corticospinal axons reach the medulla they lie within the pyramids. The pyramids are just big fiber bundles that lie on the ventral surface of the caudal medulla. The fibers in the pyramids are corticospinal. It is important to REMEMBER: THERE HAS BEEN NO CROSSING YET! in this system. The cell bodies of corticospinal axons within the pyramids lie within the IPSILATERAL cerebral cortex. Brain stem 152 Pyramids/Corticospinal Tract At the most caudal pole of the pyramids the corticospinal axons cross over the midline and now continue their descent on the contralateral (to the cell of origin) side. This crossover point is called the PYRAMIDAL DECUSSATION. The crossing fibers enter the lateral funiculus of the spinal cord where they are called the LATERAL CORTICOSPINAL TRACT (“corticospinal” is not good enough, you have to call them lateral corticospinal; LCST - remember this one??). LCST axons exit the tract to terminate upon neurons in the spinal cord gray matter along its entire length. -

Chapter 8 Nervous System
Chapter 8 Nervous System I. Functions A. Sensory Input – stimuli interpreted as touch, taste, temperature, smell, sound, blood pressure, and body position. B. Integration – CNS processes sensory input and initiates responses categorizing into immediate response, memory, or ignore C. Homeostasis – maintains through sensory input and integration by stimulating or inhibiting other systems D. Mental Activity – consciousness, memory, thinking E. Control of Muscles & Glands – controls skeletal muscle and helps control/regulate smooth muscle, cardiac muscle, and glands II. Divisions of the Nervous system – 2 anatomical/main divisions A. CNS (Central Nervous System) – consists of the brain and spinal cord B. PNS (Peripheral Nervous System) – consists of ganglia and nerves outside the brain and spinal cord – has 2 subdivisions 1. Sensory Division (Afferent) – conducts action potentials from PNS toward the CNS (by way of the sensory neurons) for evaluation 2. Motor Division (Efferent) – conducts action potentials from CNS toward the PNS (by way of the motor neurons) creating a response from an effector organ – has 2 subdivisions a. Somatic Motor System – controls skeletal muscle only b. Autonomic System – controls/effects smooth muscle, cardiac muscle, and glands – 2 branches • Sympathetic – accelerator “fight or flight” • Parasympathetic – brake “resting and digesting” * 4 Types of Effector Organs: skeletal muscle, smooth muscle, cardiac muscle, and glands. III. Cells of the Nervous System A. Neurons – receive stimuli and transmit action potentials -

The Brain Stem Medulla Oblongata
Chapter 14 The Brain Stem Medulla Oblongata Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Central sulcus Parietal lobe • embryonic myelencephalon becomes Cingulate gyrus leaves medulla oblongata Corpus callosum Parieto–occipital sulcus Frontal lobe Occipital lobe • begins at foramen magnum of the skull Thalamus Habenula Anterior Epithalamus commissure Pineal gland • extends for about 3 cm rostrally and ends Hypothalamus Posterior commissure at a groove between the medulla and Optic chiasm Mammillary body pons Cerebral aqueduct Pituitary gland Fourth ventricle Temporal lobe • slightly wider than spinal cord Cerebellum Midbrain • pyramids – pair of external ridges on Pons Medulla anterior surface oblongata – resembles side-by-side baseball bats (a) • olive – a prominent bulge lateral to each pyramid • posteriorly, gracile and cuneate fasciculi of the spinal cord continue as two pair of ridges on the medulla • all nerve fibers connecting the brain to the spinal cord pass through the medulla • four pairs of cranial nerves begin or end in medulla - IX, X, XI, XII Medulla Oblongata Associated Functions • cardiac center – adjusts rate and force of heart • vasomotor center – adjusts blood vessel diameter • respiratory centers – control rate and depth of breathing • reflex centers – for coughing, sneezing, gagging, swallowing, vomiting, salivation, sweating, movements of tongue and head Medulla Oblongata Nucleus of hypoglossal nerve Fourth ventricle Gracile nucleus Nucleus of Cuneate nucleus vagus -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Auditory and Vestibular Systems Objective • to Learn the Functional
Auditory and Vestibular Systems Objective • To learn the functional organization of the auditory and vestibular systems • To understand how one can use changes in auditory function following injury to localize the site of a lesion • To begin to learn the vestibular pathways, as a prelude to studying motor pathways controlling balance in a later lab. Ch 7 Key Figs: 7-1; 7-2; 7-4; 7-5 Clinical Case #2 Hearing loss and dizziness; CC4-1 Self evaluation • Be able to identify all structures listed in key terms and describe briefly their principal functions • Use neuroanatomy on the web to test your understanding ************************************************************************************** List of media F-5 Vestibular efferent connections The first order neurons of the vestibular system are bipolar cells whose cell bodies are located in the vestibular ganglion in the internal ear (NTA Fig. 7-3). The distal processes of these cells contact the receptor hair cells located within the ampulae of the semicircular canals and the utricle and saccule. The central processes of the bipolar cells constitute the vestibular portion of the vestibulocochlear (VIIIth cranial) nerve. Most of these primary vestibular afferents enter the ipsilateral brain stem inferior to the inferior cerebellar peduncle to terminate in the vestibular nuclear complex, which is located in the medulla and caudal pons. The vestibular nuclear complex (NTA Figs, 7-2, 7-3), which lies in the floor of the fourth ventricle, contains four nuclei: 1) the superior vestibular nucleus; 2) the inferior vestibular nucleus; 3) the lateral vestibular nucleus; and 4) the medial vestibular nucleus. Vestibular nuclei give rise to secondary fibers that project to the cerebellum, certain motor cranial nerve nuclei, the reticular formation, all spinal levels, and the thalamus. -
White Matter Tracts - Brain A143 (1)
WHITE MATTER TRACTS - BRAIN A143 (1) White Matter Tracts Last updated: August 8, 2020 CORTICOSPINAL TRACT .......................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 FUNCTION ............................................................................................................................................. 1 UNCINATE FASCICULUS ........................................................................................................................... 1 ANATOMY .............................................................................................................................................. 1 DTI PROTOCOL ...................................................................................................................................... 4 FUNCTION .............................................................................................................................................. 4 DEVELOPMENT ....................................................................................................................................... 4 CLINICAL SIGNIFICANCE ........................................................................................................................ 4 ARTICLES .............................................................................................................................................. -

Review of Spinal Cord Basics of Neuroanatomy Brain Meninges
Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Parts of Nervous System Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Neurons and Neuroglia Neuron Human brain contains per 1011-12 (trillions) neurons Body (soma) Perikaryon Nissl substance or Tigroid Dendrites Axon Myelin Terminals Synapses Neuronal types Unipolar, pseudounipolar, bipolar, multipolar Afferent (sensory, centripetal) Efferent (motor, centrifugal, effector) Associate (interneurons) Synapse Presynaptic membrane Postsynaptic membrane, receptors Synaptic cleft Synaptic vesicles, neuromediator Mitochondria In human brain – neurons 1011 (100 trillions) Synapses – 1015 (quadrillions) Neuromediators •Acetylcholine •Noradrenaline •Serotonin •GABA •Endorphin •Encephalin •P substance •Neuronal nitric oxide Adrenergic nerve ending. There are many 50-nm-diameter vesicles (arrow) with dark, electron-dense cores containing norepinephrine. x40,000. Cell Types of Neuroglia Astrocytes - Oligodendrocytes – Ependimocytes - Microglia Astrocytes – a part of hemoencephalic barrier Oligodendrocytes Ependimocytes and microglial cells Microglia represent the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors. After invading the CNS, microglial precursors disseminate relatively homogeneously throughout the neural tissue and acquire a specific phenotype, which clearly distinguish them from their precursors, the blood-derived monocytes. The ´resting´ microglia -

Memory Loss from a Subcortical White Matter Infarct
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.6.866 on 1 June 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:866-869 Short report Memory loss from a subcortical white matter infarct CAROL A KOOISTRA, KENNETH M HEILMAN From the Department ofNeurology, College ofMedicine, University ofFlorida, and the Research Service, Veterans Administration Medical Center, Gainesville, FL, USA SUMMARY Clinical disorders of memory are believed to occur from the dysfunction of either the mesial temporal lobe, the mesial thalamus, or the basal forebrain. Fibre tract damage at the level of the fornix has only inconsistently produced amnesia. A patient is reported who suffered a cerebro- vascular accident involving the posterior limb of the left internal capsule that resulted in a persistent and severe disorder of verbal memory. The inferior extent of the lesion effectively disconnected the mesial thalamus from the amygdala and the frontal cortex by disrupting the ventral amygdalofugal and thalamic-frontal pathways as they course through the diencephalon. This case demonstrates that an isolated lesion may cause memory loss without involvement of traditional structures associated with memory and may explain memory disturbances in other white matter disease such as multiple sclerosis and lacunar state. Protected by copyright. Memory loss is currently believed to reflect grey day of his illness the patient was transferred to Shands matter damage of either the mesial temporal lobe,' -4 Teaching Hospital at the University of Florida for further the mesial or the basal forebrain.'0 l evaluation. thalamus,5-9 Examination at that time showed the patient to be awake, Cerebrovascular accidents resulting in memory dys- alert, attentive and fully oriented. -

Brainstem and Its Associated Cranial Nerves
Brainstem and its Associated Cranial Nerves Anatomical and Physiological Review By Sara Alenezy With appreciation to Noura AlTawil’s significant efforts Midbrain (Mesencephalon) External Anatomy of Midbrain 1. Crus Cerebri (Also known as Basis Pedunculi or Cerebral Peduncles): Large column of descending “Upper Motor Neuron” fibers that is responsible for movement coordination, which are: a. Frontopontine fibers b. Corticospinal fibers Ventral Surface c. Corticobulbar fibers d. Temporo-pontine fibers 2. Interpeduncular Fossa: Separates the Crus Cerebri from the middle. 3. Nerve: 3rd Cranial Nerve (Oculomotor) emerges from the Interpeduncular fossa. 1. Superior Colliculus: Involved with visual reflexes. Dorsal Surface 2. Inferior Colliculus: Involved with auditory reflexes. 3. Nerve: 4th Cranial Nerve (Trochlear) emerges caudally to the Inferior Colliculus after decussating in the superior medullary velum. Internal Anatomy of Midbrain 1. Superior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). a. Tectospinal Pathway: turning the head, neck and eyeballs in response to a visual stimuli.1 Level of b. Spinotectal Pathway: turning the head, neck and eyeballs in response to a cutaneous stimuli.2 Superior 2. Oculomotor Nucleus: Situated in the periaqueductal grey matter. Colliculus 3. Red Nucleus: Red mass3 of grey matter situated centrally in the Tegmentum. Involved in motor control (Rubrospinal Tract). 1. Inferior Colliculus: Nucleus of grey matter that is associated with the Tectospinal Tract (descending) and the Spinotectal Tract (ascending). Tectospinal Pathway: turning the head, neck and eyeballs in response to a auditory stimuli. 2. Trochlear Nucleus: Situated in the periaqueductal grey matter. Level of Inferior 3. -

Cortex Brainstem Spinal Cord Thalamus Cerebellum Basal Ganglia
Harvard-MIT Division of Health Sciences and Technology HST.131: Introduction to Neuroscience Course Director: Dr. David Corey Motor Systems I 1 Emad Eskandar, MD Motor Systems I - Muscles & Spinal Cord Introduction Normal motor function requires the coordination of multiple inter-elated areas of the CNS. Understanding the contributions of these areas to generating movements and the disturbances that arise from their pathology are important challenges for the clinician and the scientist. Despite the importance of diseases that cause disorders of movement, the precise function of many of these areas is not completely clear. The main constituents of the motor system are the cortex, basal ganglia, cerebellum, brainstem, and spinal cord. Cortex Basal Ganglia Cerebellum Thalamus Brainstem Spinal Cord In very broad terms, cortical motor areas initiate voluntary movements. The cortex projects to the spinal cord directly, through the corticospinal tract - also known as the pyramidal tract, or indirectly through relay areas in the brain stem. The cortical output is modified by two parallel but separate re entrant side loops. One loop involves the basal ganglia while the other loop involves the cerebellum. The final outputs for the entire system are the alpha motor neurons of the spinal cord, also called the Lower Motor Neurons. Cortex: Planning and initiation of voluntary movements and integration of inputs from other brain areas. Basal Ganglia: Enforcement of desired movements and suppression of undesired movements. Cerebellum: Timing and precision of fine movements, adjusting ongoing movements, motor learning of skilled tasks Brain Stem: Control of balance and posture, coordination of head, neck and eye movements, motor outflow of cranial nerves Spinal Cord: Spontaneous reflexes, rhythmic movements, motor outflow to body.