Closed-Loop Neuromodulation Restores Network Connectivity And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -

Meninges,Cerebrospinal Fluid, and the Spinal Cord
The Nervous System SPINAL CORD Spinal Cord Continuation of CNS inferior to foramen magnum (medulla) Simpler Conducts impulses to and from brain Two way conduction pathway Reflex actions Spinal Cord Passes through vertebral canal Foramen magnum L2 Conus medullaris Filum terminale Cauda equina Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Spinal nerves 31 pairs Cervical and lumbar enlargements The nerves serving the upper and lower limbs emerge here Cervical Cervical spinal nerves enlargement Dura and arachnoid Thoracic mater spinal nerves Lumbar enlargement Conus medullaris Lumbar Cauda spinal nerves equina Filum (a) The spinal cord and its nerve terminale Sacral roots, with the bony vertebral spinal nerves arches removed. The dura mater and arachnoid mater are cut open and reflected laterally. Figure 12.29a Spinal Cord Protection Bone, meninges, and CSF Spinal tap-inferior to second lumbar vertebra T12 Ligamentum flavum L5 Lumbar puncture needle entering subarachnoid space L4 Supra- spinous ligament L5 Filum terminale S1 Inter- Cauda equina vertebral Arachnoid Dura in subarachnoid disc matter mater space Figure 12.30 Spinal Cord Cross section Central gray matter Cortex of white matter Epidural -

Review of Spinal Cord Basics of Neuroanatomy Brain Meninges
Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Parts of Nervous System Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Neurons and Neuroglia Neuron Human brain contains per 1011-12 (trillions) neurons Body (soma) Perikaryon Nissl substance or Tigroid Dendrites Axon Myelin Terminals Synapses Neuronal types Unipolar, pseudounipolar, bipolar, multipolar Afferent (sensory, centripetal) Efferent (motor, centrifugal, effector) Associate (interneurons) Synapse Presynaptic membrane Postsynaptic membrane, receptors Synaptic cleft Synaptic vesicles, neuromediator Mitochondria In human brain – neurons 1011 (100 trillions) Synapses – 1015 (quadrillions) Neuromediators •Acetylcholine •Noradrenaline •Serotonin •GABA •Endorphin •Encephalin •P substance •Neuronal nitric oxide Adrenergic nerve ending. There are many 50-nm-diameter vesicles (arrow) with dark, electron-dense cores containing norepinephrine. x40,000. Cell Types of Neuroglia Astrocytes - Oligodendrocytes – Ependimocytes - Microglia Astrocytes – a part of hemoencephalic barrier Oligodendrocytes Ependimocytes and microglial cells Microglia represent the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors. After invading the CNS, microglial precursors disseminate relatively homogeneously throughout the neural tissue and acquire a specific phenotype, which clearly distinguish them from their precursors, the blood-derived monocytes. The ´resting´ microglia -
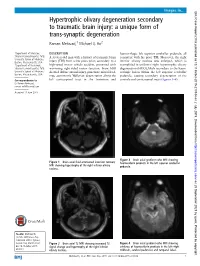
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: a Study Using a High-Resolution Semiconductor PET System
Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: A Study Using a High-Resolution Semiconductor PET System Kenji Hirata1,2, Naoya Hattori3, Wataru Takeuchi4, Tohru Shiga1, Yuichi Morimoto4, Kikuo Umegaki5, Kentaro Kobayashi1, Osamu Manabe1, Shozo Okamoto1, and Nagara Tamaki1 1Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, California; 3Department of Molecular Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 4Research and Development Group, Hitachi Ltd., Tokyo, Japan; and 5Division of Quantum Science and Engineering, Faculty of Engineering, Hokkaido University, Sapporo, Japan (1). The rubrospinal tract mainly controls limb musculature, whereas The red nucleus (RN) is a pair of small gray matter structures located the pyramidal tract can act on the whole musculature (1). A recent in the midbrain and involved in muscle movement and cognitive anatomic study indicated that a significant amount of rubrospinal tract functions. This retrospective study aimed to investigate the metabo- is present in the human brain (2). Because neuronal activity of the RN lism of human RN and its correlation to other brain regions. Methods: in Parkinson disease is known to be increased during passive and We developed a high-resolution semiconductor PET system to image voluntary movements (3), the RN may play a role in the coordination small brain structures. Twenty patients without neurologic disorders of muscular movement. Cognitive symptoms, such as intellectual underwent whole-brain scanning after injection of 400 MBq of fatigability, decreased verbal fluency, and discrete memory impair- 18F-FDG. -
Spinal Cord, Spinal Nerves, and the Autonomic Nervous System
ighapmLre21pg211_216 5/12/04 2:24 PM Page 211 impos03 302:bjighapmL:ighapmLrevshts:layouts: NAME ___________________________________ LAB TIME/DATE _______________________ REVIEW SHEET Spinal Cord, Spinal Nerves, exercise and the Autonomic Nervous System 21 Anatomy of the Spinal Cord 1. Match the descriptions given below to the proper anatomical term: Key: a. cauda equina b. conus medullaris c. filum terminale d. foramen magnum d 1. most superior boundary of the spinal cord c 2. meningeal extension beyond the spinal cord terminus b 3. spinal cord terminus a 4. collection of spinal nerves traveling in the vertebral canal below the terminus of the spinal cord 2. Match the key letters on the diagram with the following terms. m 1. anterior (ventral) hornn 6. dorsal root of spinal nervec 11. posterior (dorsal) horn k 2. arachnoid materj 7. dura materf 12. spinal nerve a 3. central canalo 8. gray commissure i 13. ventral ramus of spinal nerve h 4. dorsal ramus of spinald 9. lateral horne 14. ventral root of spinal nerve nerve g l 5. dorsal root ganglion 10. pia materb 15. white matter o a b n c m d e l f g k h j i Review Sheet 21 211 ighapmLre21pg211_216 5/12/04 2:24 PM Page 212 impos03 302:bjighapmL:ighapmLrevshts:layouts: 3. Choose the proper answer from the following key to respond to the descriptions relating to spinal cord anatomy. Key: a. afferent b. efferent c. both afferent and efferent d. association d 1. neuron type found in posterior hornb 4. fiber type in ventral root b 2. -

Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury
Neurotherapeutics (2016) 13:370–381 DOI 10.1007/s13311-016-0422-x REVIEW Reorganization of Intact Descending Motor Circuits to Replace Lost Connections After Injury Kathren L. Fink1 & William B. J. Cafferty 1 Published online: 3 February 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Neurons have a limited capacity to regenerate in the molecular mechanisms that drive plasticity within intact cir- adult central nervous system (CNS). The inability of damaged cuits is crucial in developing novel, potent, and specific ther- axons to re-establish original circuits results in permanent apeutics to restore function after SCI. In this review we dis- functional impairment after spinal cord injury (SCI). Despite cuss the evidence supporting a focus on exploring the capacity abortive regeneration of axotomized CNS neurons, limited of intact motor circuits to functionally repair the damaged spontaneous recovery of motor function emerges after partial CNS after SCI. SCI in humans and experimental rodent models of SCI. It is hypothesized that this spontaneous functional recovery is the result of the reorganization of descending motor pathways Keywords Spinal cord injury . Plasticity . Regeneration . spared by the injury, suggesting that plasticity of intact circuits Repair . Axon . Neuron is a potent alternative conduit to enhance functional recovery after SCI. In support of this hypothesis, several studies have shown that after unilateral corticospinal tract (CST) lesion (unilateral pyramidotomy), -

Cerebral Cortex
Cerebral Cortex • Research on the structure and function of the brain reveals that there are both specialized and diffuse areas of function • Motor and sensory areas are localized in discrete cortical areas called domains • Many higher mental functions such as memory and language appear to have overlapping domains and are more diffusely located • Broadmann areas are areas of localized function Cerebral Cortex - Generalizations • The cerebral cortex has three types of functional areas – Motor areas / control voluntary motor function – Sensory areas / provide conscious awareness of sensation – Association areas / act mainly to integrate diverse information for purposeful action • Each hemisphere is chiefly concerned with the sensory and motor functions of the opposite (contralateral) side of the body Motor Areas • Cortical areas controlling motor functions lie in the posterior part of the frontal lobes • Motor areas include the primary motor cortex, the premotor cortex, Broca’s area, and the front eye field Primary Motor Cortex • The primary motor cortex is located in the precentral gyrus of the frontal lobe of each hemisphere • Large neurons (pyramidal cells) in these gyri allow us to consciously control the precise or skill voluntary movements of our skeletal muscles Pyramidal cells • These long axons, which project to the spinal cord, form the massive Dendrites voluntary motor tracts called the pyramidal, or corticospinal tracts • All other descending motor tracts issue from brain stem nuclei and consists of chains of two, three, or -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study
Original Article J. Phys. Ther. Sci. 22: 7–10, 2010 Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study SUNG HO JANG, MD1), JI HEON HONG, PT, MS1), YONG HYUN KWON, PT, PhD2) 1)Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 2)Department of Physical Therapy, Yeungnam College of Science & Technology: 1737, Daemyung 7-Dong, Namgu, Daegu, 705-703, Republic of Korea. TEL +82 53-650-9702, FAX: +82 53-629-5048 Abstract. [Purpose] Little is known about the rubro-olivary tract (ROT) in the human brain. We attempted to identify the ROT using diffusion tensor tractography (DTT). [Subjects and Methods] We obtained ROT data from 11 healthy subjects with no history of neurological disorder. For tracking of the ROT, a seed region of interest (ROI) was selected in the red nucleus, and a target ROI was found in the inferior olive of each subject. [Results] The ROT, originated in the red nucleus, and passed laterally to the decussation of the superior cerebellar peduncle in the lower midbrain. In the pons, it descended through the area adjacent to the medial lemnicus in the posterior direction. Within the medulla, the ROT ended in the inferior olive, which was located lateral to the medial lemnicus and posterior to the pyramid. [Conclusion] We identified the ROT in the human brain using DTT. These results will be informative for research into the ROT in the human brain. Key words: Diffusion tensor image, Red nucleus, Inferior olive (This article was submitted Jul. 24, 2009, and was accepted Sep. -

Development of a Neuromodulation-Based Therapy for the Rehabilitation of Patients with SCI
UNIVERSIDAD DE CASTILLA LA MANCHA Escuela Internacional de Doctorado Development of a neuromodulation-based therapy for the rehabilitation of patients with SCI by Stefano Piazza A thesis submitted in the fulfillment for the degree of Doctor of Philosophy. Directors Dr. José Luis Pons Dr. Julio Gómez Soriano November 2016 i Acknowledgements A Iris, per essere sempre al mio lato, per supportarmi e incoraggiarmi, consigliarmi ed aiutarmi. Per l’energia e l’allegria, il coraggio e la passione. Perché hai aggiunto groove alla mia vita, e da allora non abbiamo piú smesso di ballare. A Mattia, che durante questa tesi sei passato da nano a gigante, per la tua simpatia e irriverenza, per le burle e per le sfide. Mi hai insegnato a non prendermi troppo sul serio ed a vedere tutte le cose con la giusta prospettiva. Ai miei genitori, sempre presenti in questi anni, avete creduto in me fin dal primo momento e non avete mai smesso di appoggiarmi. Grazie per il vostro conforto, la vostra pazienza, e per l’entusiasmo con cui avete colto ogni piccolo passo che mi avvicinava un po’ di piú a questo traguardo. Ed a Tommy, che appoggiato su una spalla non mi perde mai di vista. Alla famiglia spagnola, per accogliermi con calore, sfamarmi in svariate occasioni ed incoraggiare fin da subito tutti i nostri progetti. Ai molti buoni amici, fonte costante di gioia ed evasione, per tutti i bei momenti passati insieme, per essere sempre pronti a rallegrarmi e stimolarmi in tutti questi anni. Credo che una buona parte della mia salute mentale la debba anche a voi.