Midbrain Tegmental Lesions Affecting Or Sparing the Pupillary Fibres
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -

Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
J Neurosurg 115:971–984, 2011 Magnetic resonance imaging techniques for visualization of the subthalamic nucleus A review ELLEN J. L. BRUNENbeRG, M.SC.,1 BRAM PLATEL, PH.D.,2 PAUL A. M. HOFMAN, PH.D., M.D.,3 BART M. TER HAAR ROmeNY, PH.D.,1,4 AND VeeRLE VIsseR-VANdeWALLE, PH.D., M.D.5,6 1Department of Biomedical Engineering, Eindhoven University of Technology, Eindhoven; Departments of 3Radiology, 4Biomedical Engineering, and 5Neurosurgery, and 6Maastricht Institute for Neuromodulative Development, Maastricht University Medical Center, Maastricht, The Netherlands; and 2Fraunhofer MEVIS, Bremen, Germany The authors reviewed 70 publications on MR imaging–based targeting techniques for identifying the subtha- lamic nucleus (STN) for deep brain stimulation in patients with Parkinson disease. Of these 70 publications, 33 presented quantitatively validated results. There is still no consensus on which targeting technique to use for surgery planning; methods vary greatly between centers. Some groups apply indirect methods involving anatomical landmarks, or atlases incorporating ana- tomical or functional data. Others perform direct visualization on MR imaging, using T2-weighted spin echo or inver- sion recovery protocols. The combined studies do not offer a straightforward conclusion on the best targeting protocol. Indirect methods are not patient specific, leading to varying results between cases. On the other hand, direct targeting on MR imaging suffers from lack of contrast within the subthalamic region, resulting in a poor delineation of the STN. These defi- ciencies result in a need for intraoperative adaptation of the original target based on test stimulation with or without microelectrode recording. It is expected that future advances in MR imaging technology will lead to improvements in direct targeting. -

A Network of Genetic Repression and Derepression Specifies Projection
A network of genetic repression and derepression INAUGURAL ARTICLE specifies projection fates in the developing neocortex Karpagam Srinivasana,1, Dino P. Leonea, Rosalie K. Batesona, Gergana Dobrevab, Yoshinori Kohwic, Terumi Kohwi-Shigematsuc, Rudolf Grosschedlb, and Susan K. McConnella,2 aDepartment of Biology, Stanford University, Stanford, CA 94305; bMax-Planck Institute of Immunobiology and Epigenetics, 79108 Freiburg, Germany; and cLawrence Berkeley National Laboratory, Berkeley, CA 94720 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2011. Contributed by Susan K. McConnell, September 28, 2012 (sent for review August 24, 2012) Neurons within each layer in the mammalian cortex have stereotypic which results in a suppression of corticothalamic and callosal projections. Four genes—Fezf2, Ctip2, Tbr1, and Satb2—regulate fates, respectively, and axon extension along the corticospinal these projection identities. These genes also interact with each tract (CST). Fezf2 mutant neurons fail to repress Satb2 and Tbr1, other, and it is unclear how these interactions shape the final pro- thus their axons cross the CC and/or innervate the thalamus jection identity. Here we show, by generating double mutants of inappropriately. Fezf2, Ctip2, and Satb2, that cortical neurons deploy a complex In callosal projection neurons, Satb2 represses the expression Ctip2 Bhlhb5 genetic switch that uses mutual repression to produce subcortical of and , leading to a repression of subcerebral fates. Satb2 Tbr1 or callosal projections. We discovered that Tbr1, EphA4, and Unc5H3 Interestingly, we found that promotes expression in fi upper layer callosal neurons, and Tbr1 expression in these neu- are critical downstream targets of Satb2 in callosal fate speci ca- fi tion. -

The Superior and Inferior Colliculi of the Mole (Scalopus Aquaticus Machxinus)
THE SUPERIOR AND INFERIOR COLLICULI OF THE MOLE (SCALOPUS AQUATICUS MACHXINUS) THOMAS N. JOHNSON' Laboratory of Comparative Neurology, Departmmt of Amtomy, Un&versity of hfiehigan, Ann Arbor INTRODUCTION This investigation is a study of the afferent and efferent connections of the tectum of the midbrain in the mole (Scalo- pus aquaticus machrinus). An attempt is made to correlate these findings with the known habits of the animal. A subterranean animal of the middle western portion of the United States, Scalopus aquaticus machrinus is the largest of the genus Scalopus and its habits have been more thor- oughly studied than those of others of this genus according to Jackson ('15) and Hamilton ('43). This animal prefers a well-drained, loose soil. It usually frequents open fields and pastures but also is found in thin woods and meadows. Following a rain, new superficial burrows just below the surface of the ground are pushed in all directions to facili- tate the capture of worms and other soil life. Ten inches or more below the surface the regular permanent highway is constructed; the mole retreats here during long periods of dry weather or when frost is in the ground. The principal food is earthworms although, under some circumstances, larvae and adult insects are the more usual fare. It has been demonstrated conclusively that, under normal conditions, moles will eat vegetable matter. It seems not improbable that they may take considerable quantities of it at times. A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the University of Michigan. -
L4-Physiology of Motor Tracts.Pdf
: chapter 55 page 667 Objectives (1) Describe the upper and lower motor neurons. (2) Understand the pathway of Pyramidal tracts (Corticospinal & corticobulbar tracts). (3) Understand the lateral and ventral corticospinal tracts. (4) Explain functional role of corticospinal & corticobulbar tracts. (5) Describe the Extrapyramidal tracts as Rubrospinal, Vestibulospinal, Reticulospinal and Tectspinal Tracts. The name of the tract indicate its pathway, for example Corticobulbar : Terms: - cortico: cerebral cortex. Decustation: crossing. - Bulbar: brainstem. Ipsilateral : same side. *So it starts at cerebral cortex and Contralateral: opposite side. terminate at the brainstem. CNS influence the activity of skeletal muscle through two set of neurons : 1- Upper motor neurons (UMN) 2- lower motor neuron (LMN) They are neurons of motor cortex & their axons that pass to brain stem and They are Spinal motor neurons in the spinal spinal cord to activate: cord & cranial motor neurons in the brain • cranial motor neurons (in brainstem) stem which innervate muscles directly. • spinal motor neurons (in spinal cord) - These are the only neurons that innervate - Upper motor neurons (UMN) are the skeletal muscle fibers, they function as responsible for conveying impulses for the final common pathway, the final link voluntary motor activity through between the CNS and skeletal muscles. descending motor pathways that make up by the upper motor neurons. Lower motor neurons are classified based on the type of muscle fiber the innervate: There are two UMN Systems through which 1- alpha motor neurons (UMN) control (LMN): 2- gamma motor neurons 1- Pyramidal system (corticospinal tracts ). 2- Extrapyramidal system The activity of the lower motor neuron (LMN, spinal or cranial) is influenced by: 1. -

Dopamine Neuron Diversity: Recent Advances and Current Challenges in Human Stem Cell Models and Single Cell Sequencing
cells Review Dopamine Neuron Diversity: Recent Advances and Current Challenges in Human Stem Cell Models and Single Cell Sequencing Alessandro Fiorenzano * , Edoardo Sozzi, Malin Parmar and Petter Storm Developmental and Regenerative Neurobiology, Wallenberg Neuroscience Center, and Lund Stem Cell Centre, Department of Experimental Medical Science, Lund University, 22184 Lund, Sweden; [email protected] (E.S.); [email protected] (M.P.); [email protected] (P.S.) * Correspondence: alessandro.fi[email protected]; Tel.: +46-462220549 Abstract: Human midbrain dopamine (DA) neurons are a heterogeneous group of cells that share a common neurotransmitter phenotype and are in close anatomical proximity but display different functions, sensitivity to degeneration, and axonal innervation targets. The A9 DA neuron subtype controls motor function and is primarily degenerated in Parkinson’s disease (PD), whereas A10 neurons are largely unaffected by the condition, and their dysfunction is associated with neuropsy- chiatric disorders. Currently, DA neurons can only be reliably classified on the basis of topographical features, including anatomical location in the midbrain and projection targets in the forebrain. No systematic molecular classification at the genome-wide level has been proposed to date. Although many years of scientific efforts in embryonic and adult mouse brain have positioned us to better understand the complexity of DA neuron biology, many biological phenomena specific to humans are Citation: Fiorenzano, A.; Sozzi, E.; not amenable to being reproduced in animal models. The establishment of human cell-based systems Parmar, M.; Storm, P. Dopamine combined with advanced computational single-cell transcriptomics holds great promise for decoding Neuron Diversity: Recent Advances the mechanisms underlying maturation and diversification of human DA neurons, and linking their and Current Challenges in Human Stem Cell Models and Single Cell molecular heterogeneity to functions in the midbrain. -

Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G
Imaging of the Confused Patient: Toxic Metabolic Disorders Dara G. Jamieson, M.D. Weill Cornell Medicine, New York, NY The patient who presents with either acute or subacute confusion, in the absence of a clearly defined speech disorder and focality on neurological examination that would indicate an underlying mass lesion, needs to be evaluated for a multitude of neurological conditions. Many of the conditions that produce the recent onset of alteration in mental status, that ranges from mild confusion to florid delirium, may be due to infectious or inflammatory conditions that warrant acute intervention such as antimicrobial drugs, steroids or plasma exchange. However, some patients with recent onset of confusion have an underlying toxic-metabolic disorders indicating a specific diagnosis with need for appropriate treatment. The clinical presentations of some patients may indicate the diagnosis (e.g. hypoglycemia, chronic alcoholism) while the imaging patterns must be recognized to make the diagnosis in other patients. Toxic-metabolic disorders constitute a group of diseases and syndromes with diverse causes and clinical presentations. Many toxic-metabolic disorders have no specific neuroimaging correlates, either at early clinical stages or when florid symptoms develop. However, some toxic-metabolic disorders have characteristic abnormalities on neuroimaging, as certain areas of the central nervous system appear particularly vulnerable to specific toxins and metabolic perturbations. Areas of particular vulnerability in the brain include: 1) areas of high-oxygen demand (e.g. basal ganglia, cerebellum, hippocampus), 2) the cerebral white matter and 3) the mid-brain. Brain areas of high-oxygen demand are particularly vulnerable to toxins that interfere with cellular respiratory metabolism. -

Microstimulation of the Midbrain Tegmentum Creates Learning Signals for Saccade Adaptation
The Journal of Neuroscience, April 4, 2007 • 27(14):3759–3767 • 3759 Behavioral/Systems/Cognitive Microstimulation of the Midbrain Tegmentum Creates Learning Signals for Saccade Adaptation Yoshiko Kojima, Kaoru Yoshida, and Yoshiki Iwamoto Department of Neurophysiology, Doctoral Program in Kansei Behavioral and Brain Sciences, University of Tsukuba, Tsukuba, Ibaraki 305-8574, Japan Error signals are vital to motor learning. However, we know little about pathways that transmit error signals for learning in voluntary movements. Here we show that microstimulation of the midbrain tegmentum can induce learning in saccadic eye movements in mon- keys. Weak electrical stimuli delivered ϳ200 ms after saccades in one horizontal direction produced gradual and marked changes in saccade gain. The spatial and temporal characteristics of the produced changes were similar to those of adaptation induced by real visual error. When stimulation was applied after saccades in two different directions, endpoints of these saccades gradually shifted in the same direction in two dimensions. We conclude that microstimulation created powerful learning signals that dictate the direction of adaptive shift in movement endpoints. Our findings suggest that the error signals for saccade adaptation are conveyed in a pathway that courses through the midbrain tegmentum. Key words: motor learning; electrical stimulation; midbrain; saccade; adaptation; macaque; monkey Introduction superior colliculus, a key midbrain structure that issues saccade Motor learning ensures the accuracy of the movements we exe- signals to the premotor reticular circuitry (Scudder et al., 2002). cute daily. Vital to learning is the information about the error that The colliculus also has indirect access to the cerebellum via both results from executed movements. -
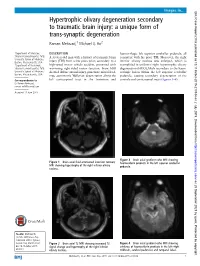
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

Brain Structure and Function Related to Headache
Review Cephalalgia 0(0) 1–26 ! International Headache Society 2018 Brain structure and function related Reprints and permissions: sagepub.co.uk/journalsPermissions.nav to headache: Brainstem structure and DOI: 10.1177/0333102418784698 function in headache journals.sagepub.com/home/cep Marta Vila-Pueyo1 , Jan Hoffmann2 , Marcela Romero-Reyes3 and Simon Akerman3 Abstract Objective: To review and discuss the literature relevant to the role of brainstem structure and function in headache. Background: Primary headache disorders, such as migraine and cluster headache, are considered disorders of the brain. As well as head-related pain, these headache disorders are also associated with other neurological symptoms, such as those related to sensory, homeostatic, autonomic, cognitive and affective processing that can all occur before, during or even after headache has ceased. Many imaging studies demonstrate activation in brainstem areas that appear specifically associated with headache disorders, especially migraine, which may be related to the mechanisms of many of these symptoms. This is further supported by preclinical studies, which demonstrate that modulation of specific brainstem nuclei alters sensory processing relevant to these symptoms, including headache, cranial autonomic responses and homeostatic mechanisms. Review focus: This review will specifically focus on the role of brainstem structures relevant to primary headaches, including medullary, pontine, and midbrain, and describe their functional role and how they relate to mechanisms -

(Rmtg),&Nbsp;A Gabaergic Afferent to Midbrain Dopamine Neurons
Neuron Article The Rostromedial Tegmental Nucleus (RMTg), a GABAergic Afferent to Midbrain Dopamine Neurons, Encodes Aversive Stimuli and Inhibits Motor Responses Thomas C. Jhou,1,* Howard L. Fields,2 Mark G. Baxter,3 Clifford B. Saper,4 and Peter C. Holland5 1Behavioral Neuroscience Branch, National Institute on Drug Abuse, Baltimore, MD 21224, USA 2Ernest Gallo Clinic and Research Center, University of California at San Francisco, Emeryville, CA 94608, USA 3Department of Experimental Psychology, University of Oxford, OX1 3UD Oxford, UK 4Beth Israel Deaconess Medical Center, Harvard University, Boston, MA 02215, USA 5Department of Psychological and Brain Sciences, Johns Hopkins University, Baltimore, MD 21218, USA *Correspondence: [email protected] DOI 10.1016/j.neuron.2009.02.001 SUMMARY vation strongly inhibits dopamine (DA) neurons (Christoph et al., 1986; Ji and Shepard, 2007), are activated by aversive Separate studies have implicated the lateral habe- stimuli and reward omission and inhibited by reward-predictive nula (LHb) or amygdala-related regions in processing cues or unexpected rewards (Matsumoto and Hikosaka, 2007, aversive stimuli, but their relationships to each other 2009; Geisler and Trimble, 2008). These ‘‘negative reward and to appetitive motivational systems are poorly prediction errors’’ are inverse to firing patterns in putative dopa- understood. We show that neurons in the recently minergic midbrain neurons (Mirenowicz and Schultz, 1994, 1996; identified GABAergic rostromedial tegmental Hollerman and Schultz, 1998; -

ON-LINE FIG 1. Selected Images of the Caudal Midbrain (Upper Row
ON-LINE FIG 1. Selected images of the caudal midbrain (upper row) and middle pons (lower row) from 4 of 13 total postmortem brains illustrate excellent anatomic contrast reproducibility across individual datasets. Subtle variations are present. Note differences in the shape of cerebral peduncles (24), decussation of superior cerebellar peduncles (25), and spinothalamic tract (12) in the midbrain of subject D (top right). These can be attributed to individual anatomic variation, some mild distortion of the brain stem during procurement at postmortem examination, and/or differences in the axial imaging plane not easily discernable during its prescription parallel to the anterior/posterior commissure plane. The numbers in parentheses in the on-line legends refer to structures in the On-line Table. AJNR Am J Neuroradiol ●:●●2019 www.ajnr.org E1 ON-LINE FIG 3. Demonstration of the dentatorubrothalamic tract within the superior cerebellar peduncle (asterisk) and rostral brain stem. A, Axial caudal midbrain image angled 10° anterosuperior to posteroinferior relative to the ACPC plane demonstrates the tract traveling the midbrain to reach the decussation (25). B, Coronal oblique image that is perpendicular to the long axis of the hippocam- pus (structure not shown) at the level of the ventral superior cerebel- lar decussation shows a component of the dentatorubrothalamic tract arising from the cerebellar dentate nucleus (63), ascending via the superior cerebellar peduncle to the decussation (25), and then enveloping the contralateral red nucleus (3). C, Parasagittal image shows the relatively long anteroposterior dimension of this tract, which becomes less compact and distinct as it ascends toward the thalamus. ON-LINE FIG 2.