Magnetic Resonance Imaging Techniques for Visualization of the Subthalamic Nucleus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MR Imaging of Ventral Thalamic Nuclei
ORIGINAL RESEARCH MR Imaging of Ventral Thalamic Nuclei K. Yamada BACKGROUND AND PURPOSE: The Vim and VPL are important target regions of the thalamus for DBS. K. Akazawa Our aim was to clarify the anatomic locations of the ventral thalamic nuclei, including the Vim and VPL, on MR imaging. S. Yuen M. Goto MATERIALS AND METHODS: Ten healthy adult volunteers underwent MR imaging by using a 1.5T S. Matsushima whole-body scanner. The subjects included 5 men and 5 women, ranging in age from 23 to 38 years, with a mean age of 28 years. The subjects were imaged with STIR sequences (TR/TE/TI ϭ 3200 ms/15 A. Takahata ms/120 ms) and DTI with a single-shot echo-planar imaging technique (TR/TE ϭ 6000 ms/88 ms, M. Nakagawa b-value ϭ 2000 s/mm2). Tractography of the CTC and spinothalamic pathway was used to identify the K. Mineura thalamic nuclei. Tractography of the PT was used as a reference, and the results were superimposed T. Nishimura on the STIR image, FA map, and color-coded vector map. RESULTS: The Vim, VPL, and PT were all in close contact at the level through the ventral thalamus. The Vim was bounded laterally by the PT and medially by the IML. The VPL was bounded anteriorly by the Vim, laterally by the internal capsule, and medially by the IML. The posterior boundary of the VPL was defined by a band of low FA that divided the VPL from the pulvinar. CONCLUSIONS: The ventral thalamic nuclei can be identified on MR imaging by using reference structures such as the PT and the IML. -

NS201C Anatomy 1: Sensory and Motor Systems
NS201C Anatomy 1: Sensory and Motor Systems 25th January 2017 Peter Ohara Department of Anatomy [email protected] The Subdivisions and Components of the Central Nervous System Axes and Anatomical Planes of Sections of the Human and Rat Brain Development of the neural tube 1 Dorsal and ventral cell groups Dermatomes and myotomes Neural crest derivatives: 1 Neural crest derivatives: 2 Development of the neural tube 2 Timing of development of the neural tube and its derivatives Timing of development of the neural tube and its derivatives Gestational Crown-rump Structure(s) age (Weeks) length (mm) 3 3 cerebral vesicles 4 4 Optic cup, otic placode (future internal ear) 5 6 cerebral vesicles, cranial nerve nuclei 6 12 Cranial and cervical flexures, rhombic lips (future cerebellum) 7 17 Thalamus, hypothalamus, internal capsule, basal ganglia Hippocampus, fornix, olfactory bulb, longitudinal fissure that 8 30 separates the hemispheres 10 53 First callosal fibers cross the midline, early cerebellum 12 80 Major expansion of the cerebral cortex 16 134 Olfactory connections established 20 185 Gyral and sulcul patterns of the cerebral cortex established Clinical case A 68 year old woman with hypertension and diabetes develops abrupt onset numbness and tingling on the right half of the face and head and the entire right hemitrunk, right arm and right leg. She does not experience any weakness or incoordination. Physical Examination: Vitals: T 37.0° C; BP 168/87; P 86; RR 16 Cardiovascular, pulmonary, and abdominal exam are within normal limits. Neurological Examination: Mental Status: Alert and oriented x 3, 3/3 recall in 3 minutes, language fluent. -
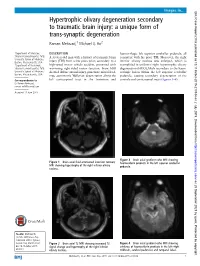
Hypertrophic Olivary Degeneration Secondary to Traumatic Brain Injury: a Unique Form of Trans-Synaptic Degeneration Raman Mehrzad,1 Michael G Ho2
… Images in BMJ Case Reports: first published as 10.1136/bcr-2015-210334 on 2 July 2015. Downloaded from Hypertrophic olivary degeneration secondary to traumatic brain injury: a unique form of trans-synaptic degeneration Raman Mehrzad,1 Michael G Ho2 1Department of Medicine, DESCRIPTION haemorrhagic left superior cerebellar peduncle, all Steward Carney Hospital, Tufts A 33-year-old man with a history of traumatic brain consistent with his prior TBI. Moreover, the right University School of Medicine, Boston, Massachusetts, USA injury (TBI) from a few years prior, secondary to a inferior olivary nucleus was enlarged, which is 2Department of Neurology, high-speed motor vehicle accident, presented with exemplified in unilateral right hypertrophic olivary Steward Carney Hospital, Tufts worsening right-sided motor function. Brain MRI degeneration (HOD), likely secondary to the haem- University School of Medicine, showed diffuse axonal injury, punctuate microbleed- orrhagic lesion within the left superior cerebellar Boston, Massachusetts, USA ings, asymmetric Wallerian degeneration along the peduncle, causing secondary degeneration of the fi – Correspondence to left corticospinal tract in the brainstem and contralateral corticospinal tracts ( gures 1 6). Dr Raman Mehrzad, [email protected] Accepted 11 June 2015 http://casereports.bmj.com/ fl Figure 3 Brain axial gradient echo MRI showing Figure 1 Brain axial uid-attenuated inversion recovery haemosiderin products in the left superior cerebellar MRI showing hypertrophy of the right inferior olivary peduncle. nucleus. on 25 September 2021 by guest. Protected copyright. To cite: Mehrzad R, Ho MG. BMJ Case Rep Published online: [please include Day Month Year] Figure 2 Brain axial T2 MRI showing increased T2 Figure 4 Brain axial gradient echo MRI showing doi:10.1136/bcr-2015- signal change and hypertrophy of the right inferior evidence of haemosiderin products in the left>right 210334 olivary nucleus. -

ON-LINE FIG 1. Selected Images of the Caudal Midbrain (Upper Row
ON-LINE FIG 1. Selected images of the caudal midbrain (upper row) and middle pons (lower row) from 4 of 13 total postmortem brains illustrate excellent anatomic contrast reproducibility across individual datasets. Subtle variations are present. Note differences in the shape of cerebral peduncles (24), decussation of superior cerebellar peduncles (25), and spinothalamic tract (12) in the midbrain of subject D (top right). These can be attributed to individual anatomic variation, some mild distortion of the brain stem during procurement at postmortem examination, and/or differences in the axial imaging plane not easily discernable during its prescription parallel to the anterior/posterior commissure plane. The numbers in parentheses in the on-line legends refer to structures in the On-line Table. AJNR Am J Neuroradiol ●:●●2019 www.ajnr.org E1 ON-LINE FIG 3. Demonstration of the dentatorubrothalamic tract within the superior cerebellar peduncle (asterisk) and rostral brain stem. A, Axial caudal midbrain image angled 10° anterosuperior to posteroinferior relative to the ACPC plane demonstrates the tract traveling the midbrain to reach the decussation (25). B, Coronal oblique image that is perpendicular to the long axis of the hippocam- pus (structure not shown) at the level of the ventral superior cerebel- lar decussation shows a component of the dentatorubrothalamic tract arising from the cerebellar dentate nucleus (63), ascending via the superior cerebellar peduncle to the decussation (25), and then enveloping the contralateral red nucleus (3). C, Parasagittal image shows the relatively long anteroposterior dimension of this tract, which becomes less compact and distinct as it ascends toward the thalamus. ON-LINE FIG 2. -

Multistable Properties of Human Subthalamic Nucleus Neurons in Parkinson’S Disease
Multistable properties of human subthalamic nucleus neurons in Parkinson’s disease Jeremy W. Chopeka,1, Hans Hultbornb, and Robert M. Brownstonea,2 aDepartment of Neuromuscular Diseases, UCL Queen Square Institute of Neurology, University College London, WC1N 3BG London, United Kingdom; and bDepartment of Neuroscience, University of Copenhagen, 2200 Copenhagen N, Denmark Edited by Peter L. Strick, University of Pittsburgh, Pittsburgh, PA, and approved October 15, 2019 (received for review July 18, 2019) To understand the function and dysfunction of neural circuits, it is thorough characterization of complex neuronal properties is necessary to understand the properties of the neurons participating critical for understanding the modus operandi of neural circuits. in the behavior, the connectivity between these neurons, and the The connectivity of the excitatory subthalamic nucleus (STN) neuromodulatory status of the circuits at the time they are producing of the basal ganglia is well understood: it receives inputs from the the behavior. Such knowledge of human neural circuits is difficult, globus pallidus externa (GPe), motor cortex, and substantia nigra at best, to obtain. Here, we study firing properties of human pars compacta, and projects to the GPe, globus pallidus interna, subthalamic neurons, using microelectrode recordings and microstim- and substantia nigra pars reticulata. Furthermore, the basic ulation during awake surgery for Parkinson’s disease. We dem- electrophysiological properties of these neurons is reasonably onstrate that low-amplitude, brief trains of microstimulation can lead well understood, with resurgent and persistent sodium- and to persistent changes in neuronal firing behavior including switching calcium-dependent potassium conductances playing key roles for between firing rates, entering silent periods, or firing several bursts repetitive firing, and low-threshold calcium currents playing a then entering a silent period. -

Closed-Loop Neuromodulation Restores Network Connectivity And
RESEARCH ARTICLE Closed-loop neuromodulation restores network connectivity and motor control after spinal cord injury Patrick D Ganzer1,2*, Michael J Darrow1, Eric C Meyers1,2, Bleyda R Solorzano2, Andrea D Ruiz2, Nicole M Robertson2, Katherine S Adcock3, Justin T James2, Han S Jeong2, April M Becker4, Mark P Goldberg4, David T Pruitt1,2, Seth A Hays1,2,3, Michael P Kilgard1,2,3, Robert L Rennaker II1,2,3* 1Erik Jonsson School of Engineering and Computer Science, The University of Texas at Dallas, Richardson, United States; 2Texas Biomedical Device Center, Richardson, United States; 3School of Behavioral Brain Sciences, The University of Texas at Dallas, Richardson, United States; 4Department of Neurology and Neurotherapeutics, University of Texas Southwestern Medical Center, Dallas, United States Abstract Recovery from serious neurological injury requires substantial rewiring of neural circuits. Precisely-timed electrical stimulation could be used to restore corrective feedback mechanisms and promote adaptive plasticity after neurological insult, such as spinal cord injury (SCI) or stroke. This study provides the first evidence that closed-loop vagus nerve stimulation (CLV) based on the synaptic eligibility trace leads to dramatic recovery from the most common forms of SCI. The addition of CLV to rehabilitation promoted substantially more recovery of forelimb function compared to rehabilitation alone following chronic unilateral or bilateral cervical SCI in a rat model. Triggering stimulation on the most successful movements is critical to maximize recovery. CLV enhances recovery by strengthening synaptic connectivity from remaining motor *For correspondence: networks to the grasping muscles in the forelimb. The benefits of CLV persist long after the end of [email protected] stimulation because connectivity in critical neural circuits has been restored. -

Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: a Study Using a High-Resolution Semiconductor PET System
Metabolic Activity of Red Nucleus and Its Correlation with Cerebral Cortex and Cerebellum: A Study Using a High-Resolution Semiconductor PET System Kenji Hirata1,2, Naoya Hattori3, Wataru Takeuchi4, Tohru Shiga1, Yuichi Morimoto4, Kikuo Umegaki5, Kentaro Kobayashi1, Osamu Manabe1, Shozo Okamoto1, and Nagara Tamaki1 1Department of Nuclear Medicine, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 2Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, California; 3Department of Molecular Imaging, Hokkaido University Graduate School of Medicine, Sapporo, Japan; 4Research and Development Group, Hitachi Ltd., Tokyo, Japan; and 5Division of Quantum Science and Engineering, Faculty of Engineering, Hokkaido University, Sapporo, Japan (1). The rubrospinal tract mainly controls limb musculature, whereas The red nucleus (RN) is a pair of small gray matter structures located the pyramidal tract can act on the whole musculature (1). A recent in the midbrain and involved in muscle movement and cognitive anatomic study indicated that a significant amount of rubrospinal tract functions. This retrospective study aimed to investigate the metabo- is present in the human brain (2). Because neuronal activity of the RN lism of human RN and its correlation to other brain regions. Methods: in Parkinson disease is known to be increased during passive and We developed a high-resolution semiconductor PET system to image voluntary movements (3), the RN may play a role in the coordination small brain structures. Twenty patients without neurologic disorders of muscular movement. Cognitive symptoms, such as intellectual underwent whole-brain scanning after injection of 400 MBq of fatigability, decreased verbal fluency, and discrete memory impair- 18F-FDG. -

Motor Systems Basal Ganglia
Motor systems 409 Basal Ganglia You have just read about the different motor-related cortical areas. Premotor areas are involved in planning, while MI is involved in execution. What you don’t know is that the cortical areas involved in movement control need “help” from other brain circuits in order to smoothly orchestrate motor behaviors. One of these circuits involves a group of structures deep in the brain called the basal ganglia. While their exact motor function is still debated, the basal ganglia clearly regulate movement. Without information from the basal ganglia, the cortex is unable to properly direct motor control, and the deficits seen in Parkinson’s and Huntington’s disease and related movement disorders become apparent. Let’s start with the anatomy of the basal ganglia. The important “players” are identified in the adjacent figure. The caudate and putamen have similar functions, and we will consider them as one in this discussion. Together the caudate and putamen are called the neostriatum or simply striatum. All input to the basal ganglia circuit comes via the striatum. This input comes mainly from motor cortical areas. Notice that the caudate (L. tail) appears twice in many frontal brain sections. This is because the caudate curves around with the lateral ventricle. The head of the caudate is most anterior. It gives rise to a body whose “tail” extends with the ventricle into the temporal lobe (the “ball” at the end of the tail is the amygdala, whose limbic functions you will learn about later). Medial to the putamen is the globus pallidus (GP). -

The Subthalamic Nucleus
The Subthalamic Nucleus Part I: Development, Cytology, Topography and Connections Bearbeitet von Enrico Marani, Tjitske Heida, Egbert A. J. F Lakke, Kamen G Usunoff 1. Auflage 2008. Taschenbuch. xiv, 117 S. Paperback ISBN 978 3 540 79459 2 Format (B x L): 15,5 x 23,5 cm Gewicht: 213 g Weitere Fachgebiete > Psychologie > Allgemeine Psychologie / Grundlagenfächer > Biologische Psychologie, Neuropsychologie, Psychophysiologie schnell und portofrei erhältlich bei Die Online-Fachbuchhandlung beck-shop.de ist spezialisiert auf Fachbücher, insbesondere Recht, Steuern und Wirtschaft. Im Sortiment finden Sie alle Medien (Bücher, Zeitschriften, CDs, eBooks, etc.) aller Verlage. Ergänzt wird das Programm durch Services wie Neuerscheinungsdienst oder Zusammenstellungen von Büchern zu Sonderpreisen. Der Shop führt mehr als 8 Millionen Produkte. 76 Nigro-Subthalamic Connections in the Rat Cossette et al. (1999), Francois et al. (2000) and Hedreen (1999). An overview of the dopaminergic innervation in the basal ganglia is given by Smith and Kievel (2000). 6 Nigro-Subthalamic Connections in the Rat 6.1 Introduction The STN projection neurons are glutamatergic, excitatory, and heavily inner- vated by widely branching axons of the substantia nigra (SN) (see Sects. 5.1 and 5.2.10, this volume). Leucine-labelled fibres of the STN follow in their projections the laminar organization of the substantia nigra’s pars reticulata (Tokuno et al. 1990). However, the nigro-subthalamic connection remained controversial (see Sect. 5.2.10, this volume) due to its incomplete description in various experimen- tal animals. Although functional dopamine receptors are expressed in the STN (see Sect. 2.3.4.1, this volume), the direct modulation of subthalamic neurons by dopamine of the substantia nigra is controversial owing to the low density of dopamine axons in the STN (see Cragg et al. -

Compensatory Sprouting and Impulse Rerouting After Unilateral Pyramidal Tract Lesion in Neonatal Rats
The Journal of Neuroscience, September 1, 2000, 20(17):6561–6569 Compensatory Sprouting and Impulse Rerouting after Unilateral Pyramidal Tract Lesion in Neonatal Rats Werner J. Z’Graggen, Karim Fouad, Olivier Raineteau, Gerlinde A. S. Metz, Martin E. Schwab, and Gwendolyn L. Kartje Brain Research Institute, University of Zurich and Swiss Federal Institute of Technology Zurich, CH-8057 Zurich, Switzerland After lesions of the developing mammalian CNS, structural plas- tigate possible new functional connections from the motor cortex ticity and functional recovery are much more pronounced than in of the pyramidotomy side to the periphery. In rats lesioned as the mature CNS. We investigated the anatomical reorganization adults, stimulation of the motor cortex ipsilateral to the pyra- of the corticofugal projections rostral to a unilateral lesion of the midotomy never elicited EMG activity. In contrast, in P2 lesioned corticospinal tract at the level of the medullary pyramid (pyra- rats bilateral forelimb EMGs were found. EMG latencies were midotomy) and the contribution of this reorganization and other comparable for the ipsilateral and contralateral responses but descending systems to functional recovery. were significantly longer than in unlesioned animals. Transient Two-day-old (P2) and adult rats underwent a unilateral pyra- inactivation of both red nuclei with the GABA receptor agonist midotomy. Three months later the corticofugal projections to the muscimol led to a complete loss of these bilateral movements. red nucleus and the pons were analyzed; a relatively large num- Movements and EMGs reappeared after wash-out of the drug. ber of corticorubral and corticopontine fibers from the lesioned These results suggest an important role of the red nucleus in the side had crossed the midline and established an additional con- tralateral innervation of the red nucleus and the pons. -

Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study
Original Article J. Phys. Ther. Sci. 22: 7–10, 2010 Identification of the Rubro-Olivary Tract in the Human Brain: a Diffusion Tensor Tractography Study SUNG HO JANG, MD1), JI HEON HONG, PT, MS1), YONG HYUN KWON, PT, PhD2) 1)Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University 2)Department of Physical Therapy, Yeungnam College of Science & Technology: 1737, Daemyung 7-Dong, Namgu, Daegu, 705-703, Republic of Korea. TEL +82 53-650-9702, FAX: +82 53-629-5048 Abstract. [Purpose] Little is known about the rubro-olivary tract (ROT) in the human brain. We attempted to identify the ROT using diffusion tensor tractography (DTT). [Subjects and Methods] We obtained ROT data from 11 healthy subjects with no history of neurological disorder. For tracking of the ROT, a seed region of interest (ROI) was selected in the red nucleus, and a target ROI was found in the inferior olive of each subject. [Results] The ROT, originated in the red nucleus, and passed laterally to the decussation of the superior cerebellar peduncle in the lower midbrain. In the pons, it descended through the area adjacent to the medial lemnicus in the posterior direction. Within the medulla, the ROT ended in the inferior olive, which was located lateral to the medial lemnicus and posterior to the pyramid. [Conclusion] We identified the ROT in the human brain using DTT. These results will be informative for research into the ROT in the human brain. Key words: Diffusion tensor image, Red nucleus, Inferior olive (This article was submitted Jul. 24, 2009, and was accepted Sep. -

Independent Circuits in the Basal Ganglia for the Evaluation And
Independent circuits in the basal ganglia for the PNAS PLUS evaluation and selection of actions Marcus Stephenson-Jones1,2, Andreas A. Kardamakis, Brita Robertson, and Sten Grillner2 Department of Neuroscience, Karolinska Institutet, SE-171 77 Stockholm, Sweden Contributed by Sten Grillner, August 8, 2013 (sent for review June 7, 2013) The basal ganglia are critical for selecting actions and evaluating (17). These results show that the pallidal neurons projecting to the their outcome. Although the circuitry for selection is well un- habenula encode information about the expected and achieved derstood, how these nuclei evaluate the outcome of actions is value of an action. unknown. Here, we show in lamprey that a separate evaluation Consequently, it appears that separate populations of neurons in circuit, which regulates the habenula-projecting globus pallidus the globus pallidus are involved in the selection (brainstem/tha- (GPh) neurons, exists within the basal ganglia. The GPh neurons lamic projecting) and evaluation (habenula-projecting) of actions. are glutamatergic and can drive the activity of the lateral This raises the possibility that independent circuits within the basal fl habenula, which, in turn, provides an indirect inhibitory in uence ganglia control these globus pallidus populations to regulate action on midbrain dopamine neurons. We show that GPh neurons selection or evaluation. Although the selection circuitry, as men- receive inhibitory input from the striosomal compartment of the tioned above, is described in detail, no studies have determined the striatum. The striosomal input can reduce the excitatory drive to evaluation circuitry within the basal ganglia that provides the the lateral habenula and, consequently, decrease the inhibition onto the dopaminergic system.